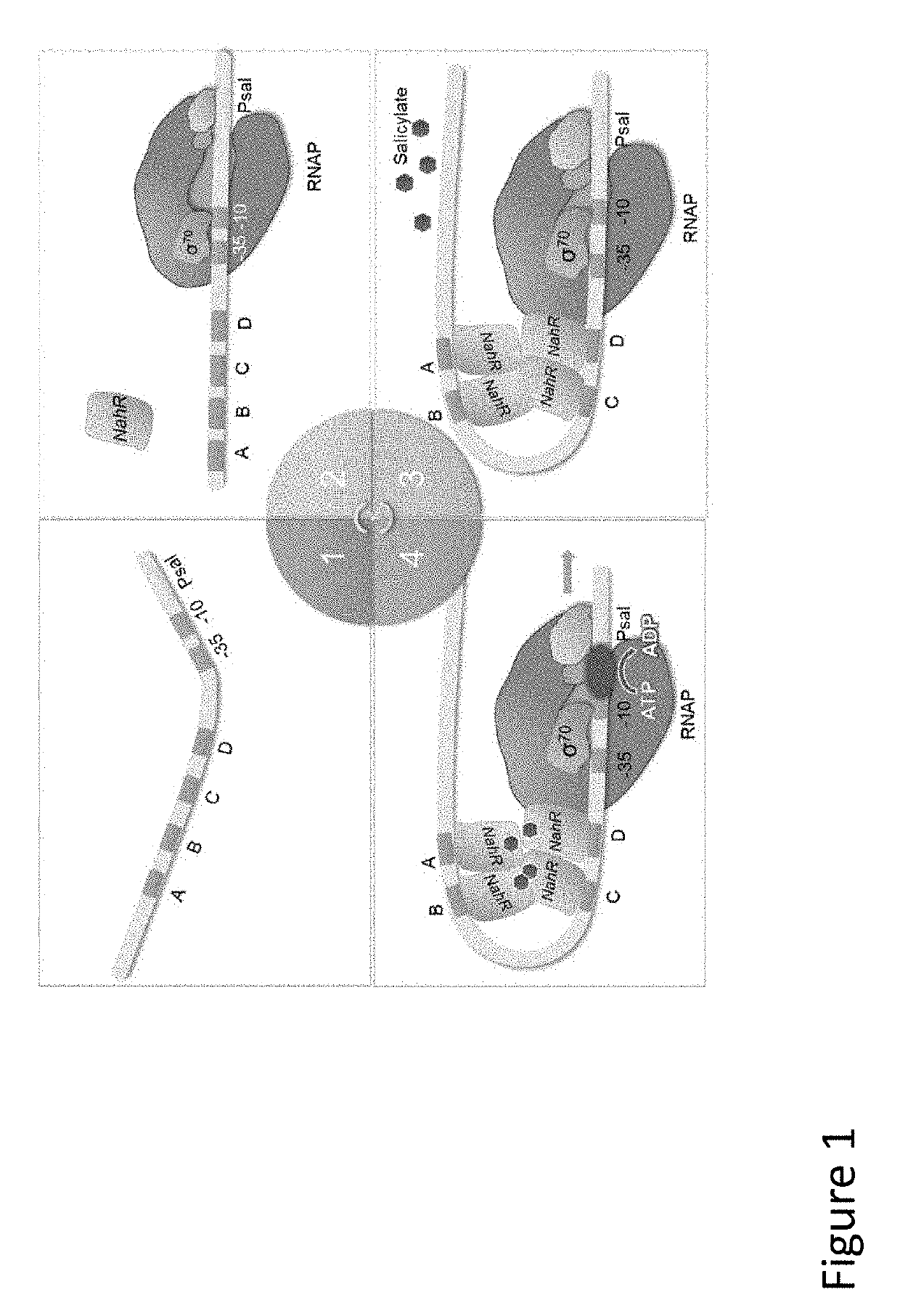
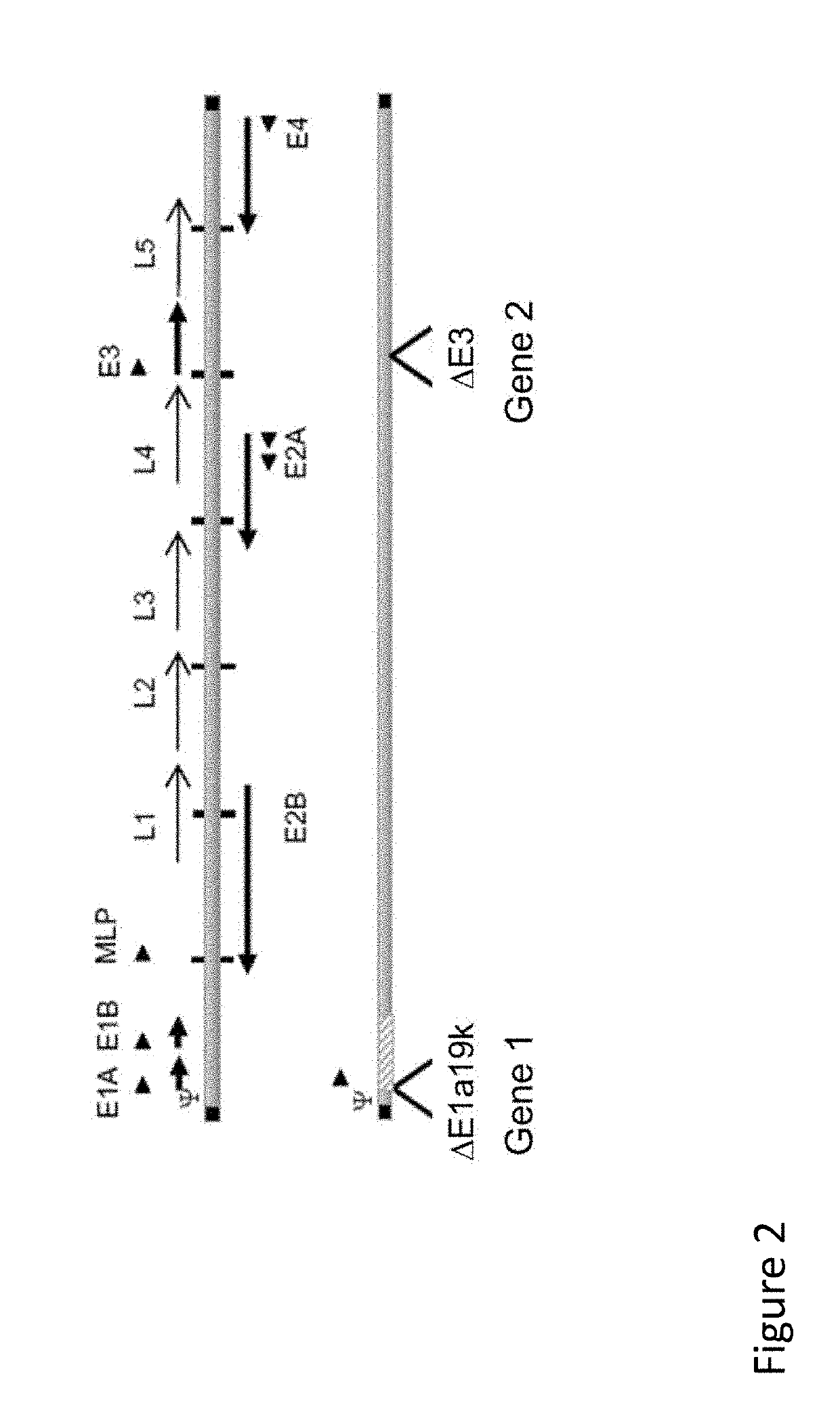
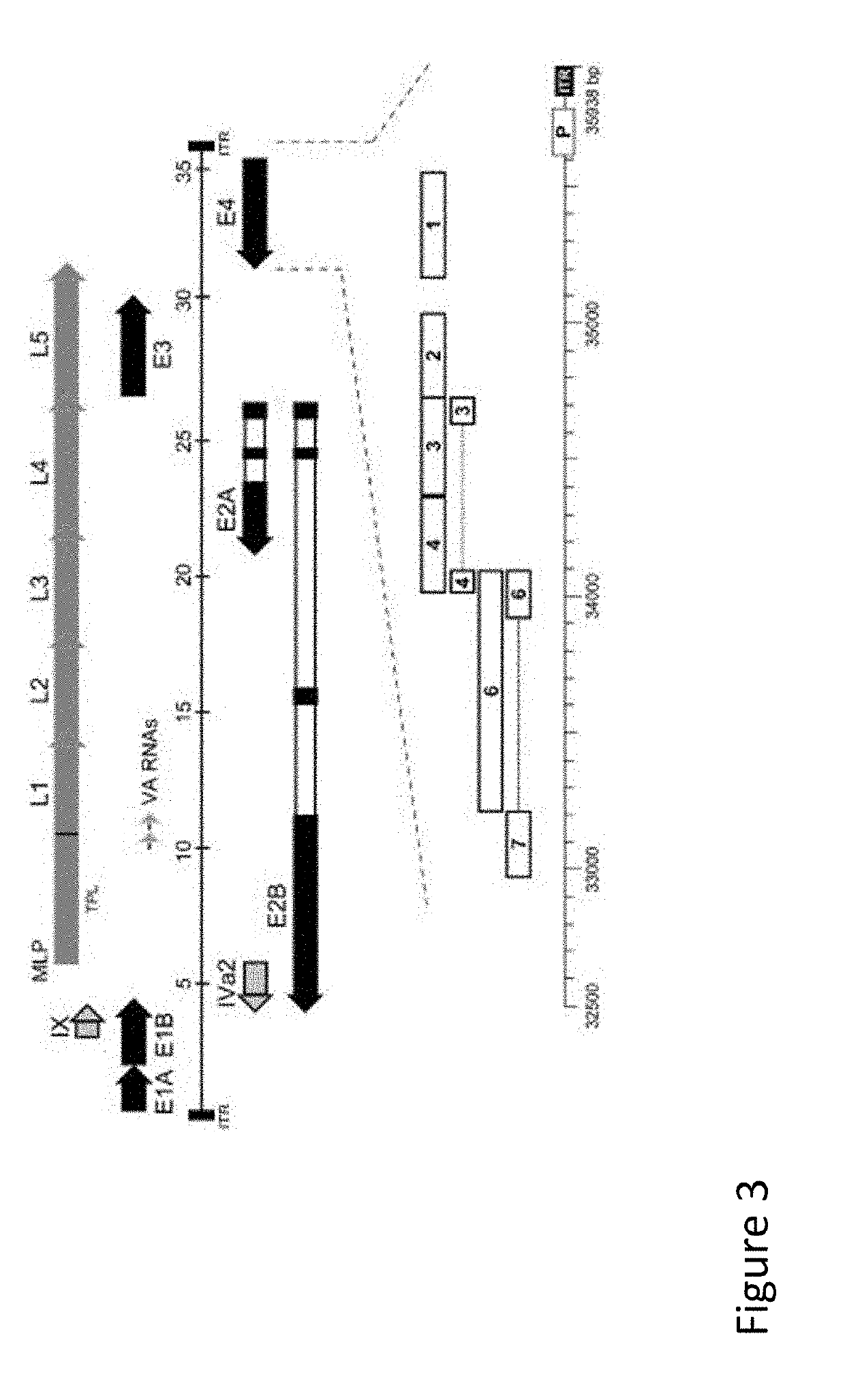
Immunomodulatory Oncolytic Adenoviral Vectors, and Methods of Production and Use Thereof for Treatment of Cancer
a technology of adenovirus and adenovirus, which is applied in the field of immunomodulatory oncolytic adenovirus, can solve the problems of reduced ability to propagate and lyse tumor cells compared to wild-type virus infections, high transgene expression, and often fail treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
and Recombinant Nucleic Acids
[0186]Standard techniques are used for recombinant nucleic acid methods, polynucleotide synthesis, and microbial culture and transformation (e.g., electroporation, lipofection). Generally, enzymatic reactions and purification steps are performed according to the manufacturer's specifications. The techniques and procedures are generally performed according to conventional methods in the art and various general references (see generally, Sambrook et al., Molecular Cloning: A Laboratory Manual, 2nd. edition (1989) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.), which are provided throughout this document. The nomenclature used herein and the laboratory procedures in analytical chemistry, organic synthetic chemistry, and pharmaceutical formulation and delivery, and treatment of patients. Methods for the construction of adenoviral mutants are generally known in the art. See Bett, A. J. et al, PNAS 1994 vol: 91, pages 8802-8806, Mittal, S. K., ...
example 2
tion
[0189]Initial screening of recombinant virus was based on isolated viral DNA rescued from transfected 293 cells where viral propagation based cytopathic effects (CPE) were observed. Viral DNA was used as a template for PCR based detection of sequences flanking the site of the TAV-255 E1a enhancer deletion region. Wild-type sequence would produce a band of 350 bp, while DNA which had the E1a enhancer deletion would only generate a clearly distinguishable 300 bp band. In addition, primers specific for internal sequences of the IL-12 transgene and for Ad hexon sequences were used to verify 400 bp and 3 kb PCR amplified bands respectively that would only be present in viral DNA generated by recombination between the two parental plasmids containing either the E1 region and transgene insert or the late Ad structural proteins derived solely from the pAdEasy™ plasmid. Individual constructs were isolated by two rounds of plaque purification on A549 or 293 cells using standard methods (T...
example 3
[0190]Tumor specific viral lysis was evaluated in both tumor and non-tumor cell lines of murine and human origin by infection of the cells in vitro with the viruses, followed by standard crystal violet for cell viability over time as instructed in the kit manuals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
| compositions | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com