Method for detecting entry of small molecule compounds into mitochondria at cell in-situ level
A technology of small molecule compounds and mitochondria, which is applied in the field of molecular pharmacology, can solve the problems that cannot explain the way small molecule drugs enter the mitochondria, and achieve the effect of no background interference, high sensitivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of the small molecule compound (hereinafter represented by NA17) of structure shown in formula (A)
[0039] The synthetic route of NA17 is shown below, and the reagents and conditions are as follows: a: piperethylamine, ethanol, 70°C, stirred for 10 hours; b: propylamine, DMSO, 135°C:
[0040]
[0041] The compound (2.77g, 10mmol) of the structure shown in the formula (B) was dissolved in 150ml ethanol (100v / v%), fully stirred, then added piperonyl ethylamine (1.65g, 10mmol), and the reaction mixture was heated at 70°C The reaction was stirred for 10 hours. After the reaction was over, the solution was cooled to room temperature and filtered, and the filter cake was collected to obtain an intermediate product of the structure shown in formula (C), which was directly used in the next step without further purification; (C) The intermediate product (4.23g, 10mmol) and 3-dimethylaminopropylamine (1.02g, 10mmol) of the structure shown in (C) ...
Embodiment 2
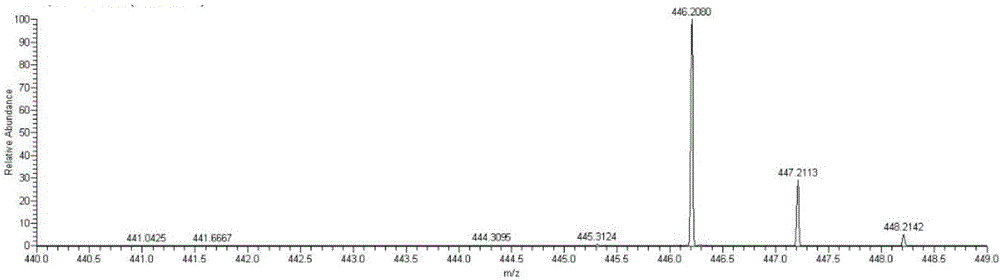
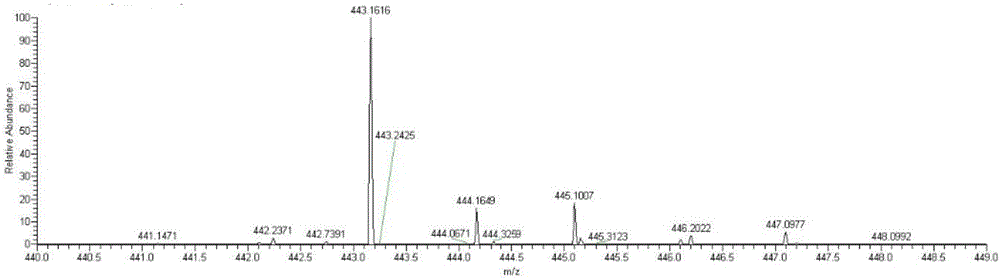
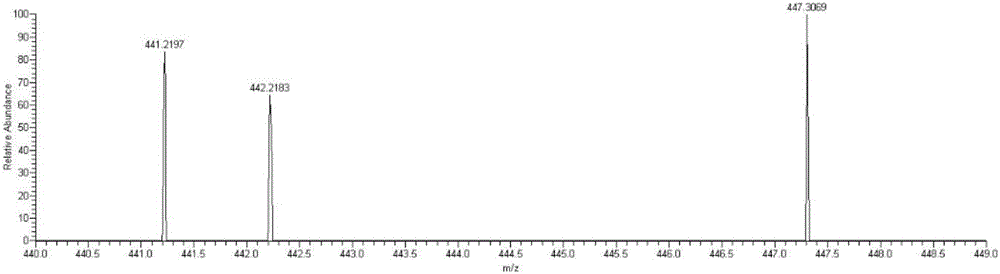
[0043] Example 2: Detecting whether NA17 can enter mitochondria at the in situ level of cells
[0044] (1) Cell administration
[0045] 1. Inoculate the non-small cell lung cancer cell line NCI-H460 on a 70cm culture dish, carefully remove the medium in the culture dish when the cell confluence reaches 50% to 60%, wash twice with PBS, and add new DMEM culture medium .
[0046] 2. 2 mM NA17 original drug was prepared with DMSO as a solvent, added to a petri dish so that the final concentration was 10 μM, administered for 5 hours, and a control group without drug addition was set at the same time.
[0047] (2) Separation of mitochondria
[0048] 1. Carefully remove the medium in the culture dish after the administration treatment, wash twice with PBS, digest the adherent cells with 0.25% trypsin; add 5mL of PBS solution, blow off the adherent cells and collect the suspension in a 10mL centrifuge centrifuge at 2000rpm for 10min in a centrifuge;
[0049] 2. Discard the superna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com