Brucella abortus reference serum bank
A technology of bovine Brucella and Brucella, which is applied in the field of bovine brucellosis reference serum bank, can solve the problems of interfering with guinea pig complement, difficulty in universal use, and difficulty in the preparation of hemolysin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] ——Preparation of clinical positive reference serum
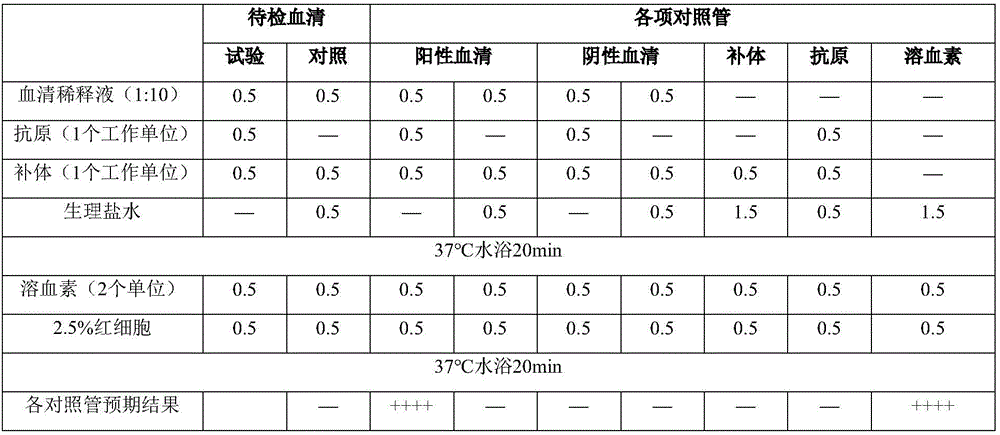
[0033]1. Collect more than 300 samples of serum from dairy cows with clinical history of abortion and not immunized with vaccines, and use tiger bengal plate agglutination test for preliminary determination. Take 0.03ml of the serum to be tested and the same amount of Brucella tiger red plate agglutination test antigen (batch number: 201501) for plate agglutination, and observe the reaction result within 4 minutes. Results A total of 79 positive sera were detected by the tiger bengal test.
[0034] 2. For the 79 samples that were tested positive for tiger bengal, the antibody titer of Brucella was further determined by test tube agglutination test, and the serum samples with antibody titer above 1:100 were reserved for further complement fixation test. The specific operation is as follows: the test tube agglutinated antigen (batch 201501) was diluted 1:20 with 0.5% phenol saline (abbreviation: stone saline); the seru...
Embodiment 2
[0047] ——The results of 79 sera measured by three different detection methods.
[0048] The 79 bovine sera that were initially screened positive by tiger bengal were further tested by test tube agglutination test and complement fixation test, and the sera that were tested positive by all three methods were used as positive reference serum samples for Brucella bovis, and were accurately packaged lyophilized. The detection results of 79 serums by three different methods are as follows (Table 4):
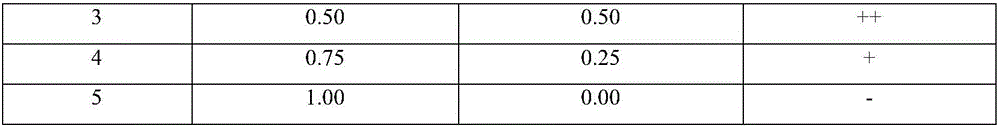
[0049] Table 4 The results of clinically collected Brucella bovis positive serum using three different detection methods
[0050]
[0051]
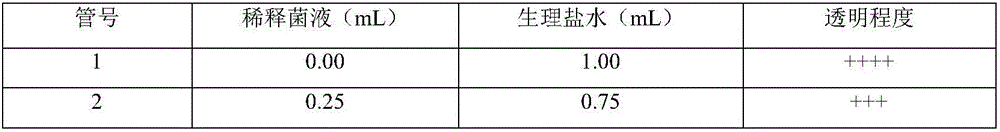
[0052] Note: 1. RBT: tiger bengal plate agglutination test, take 0.03ml of the serum to be tested and the same amount of Brucella tiger bengal plate agglutination test antigen (batch number: 201501) for plate agglutination, and observe the reaction result within 4 minutes; + +++: There are large agglutinated particles or particles, and the...
Embodiment 3
[0061] ——Preparation of vaccine immune serum
[0062] Track and monitor the antibody level of immune flocks, and collect serum as the source of immune positive serum samples. Adopt brucellosis vaccine (S2 strain) to carry out oral immunization to test sheep and goat, 2.5 billion CFU / head portion. After the completion of immunization, the antibody level was monitored weekly by tiger bengal plate agglutination test, and part of the bovine serum was collected when the antibody level was high, and titer determination and complement fixation test were confirmed according to the preparation method of clinical positive serum samples. Each serum 0.5ml / cartridge was aliquoted and freeze-dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com