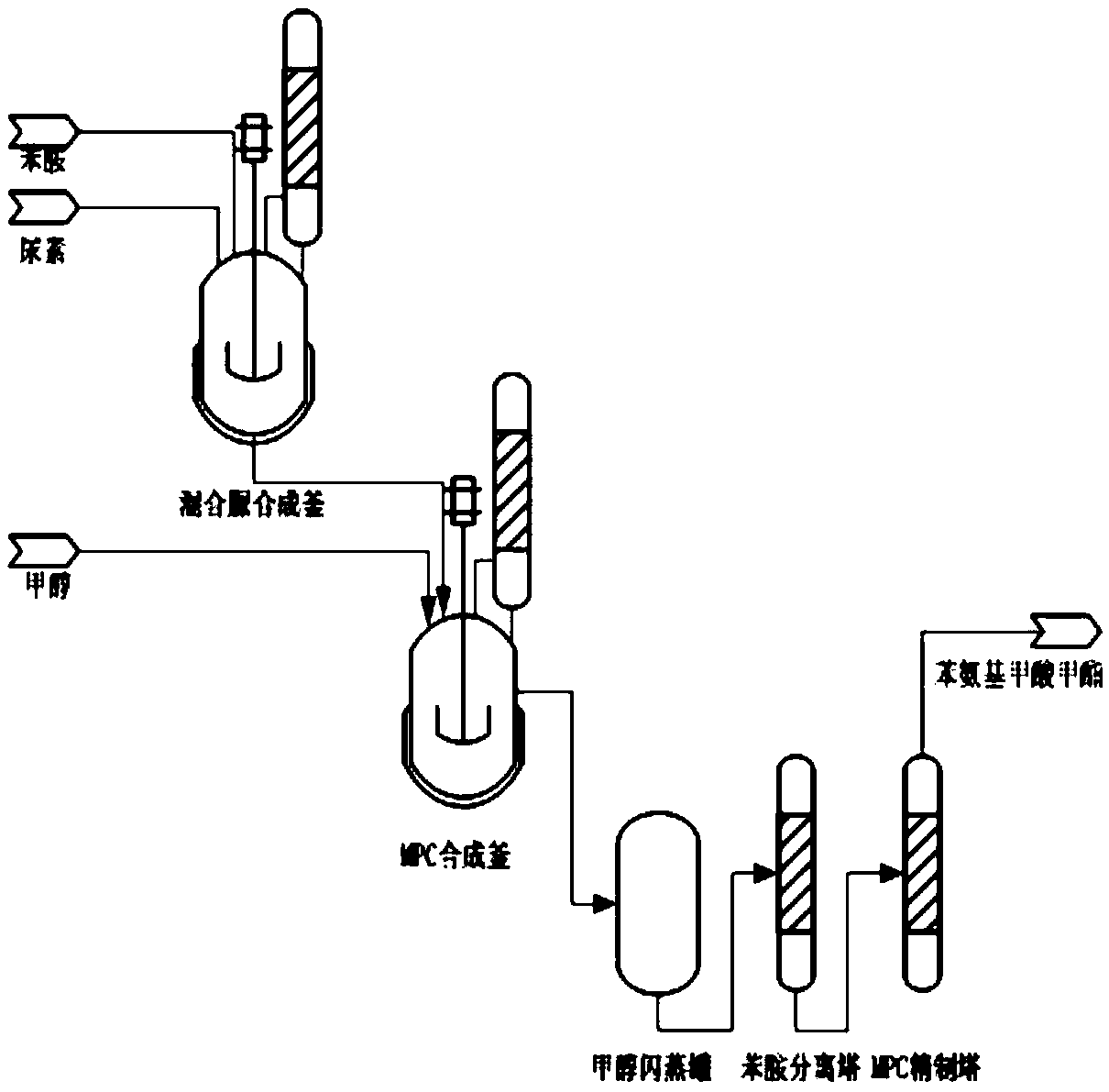
A kind of method of directly synthesizing methyl phenylcarbamate by aniline and urea
A technology of methyl phenylcarbamate and urea, which is applied in the field of synthesis of methyl phenylcarbamate, can solve the problems of low degree of automation, high energy consumption, high cost, etc., achieve simplified operation process, overcome product yield, and be conducive to industrialization The effect of promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 42.4g of urea and 157.6g of aniline were added to a 1L high-pressure reaction separation device for reaction. After the reaction, 138.7g of reaction product A was collected. The reaction product A was analyzed by HPLC to obtain the mass percentage of each component. Phenylurea is 22.7%, diphenylurea is 71.8%, aniline is 5.1%, aniline oxide and ammonium carbonate are less than 0.1%; 90g methanol is weighed into the above reaction product A and mixed uniformly, and then 300g chlorobenzene is added , The reaction device was sealed, and nitrogen gas with a flow rate of 200 ml / min was introduced into it, and the pressure of the reaction device was controlled to 2.3 MPa and heated to 175°C. Stay at 175°C for 40 minutes, stop heating, and cool down naturally to room temperature. Finally, open the device and collect 268.5g of reaction product B. Perform high performance liquid phase analysis on reaction product B. The content of each component in the product is monophenylurea. U...
Embodiment 2
[0029] 60.0g of urea and 204.6g of aniline were added to the 1L high-pressure reaction separation device for reaction. After the reaction, 189.5g of reaction product A was collected. The reaction product A was analyzed by high performance liquid phase, and the mass percentage of monophenylurea was 28.7% , The mass percentage of diphenylurea is 66.8%, and the mass percentage of aniline is 4.3%. Weigh 63.8g of methanol and add it to the above reaction product A and mix evenly, then add 300g of o-dichlorobenzene, seal the reaction device, pass nitrogen gas at a flow rate of 10ml / min into it, and control the pressure of the reaction device to 2.5MPa, And heated to 175°C. Stay at 175°C for 40 minutes, stop heating, and naturally cool to room temperature. Finally, open the device and collect 548.3g of reaction product B. The reaction product B is analyzed by HPLC. Among them, monophenylurea and diphenylurea are undetectable. Urea accounted for 0.21%, methyl phenylcarbamate accounted...
Embodiment 3
[0032] 50.0 g of urea and 186.0 g of aniline were added to a 1L high-pressure reaction separation device for reaction. After the reaction, 174.2 g of reaction product A was collected, and the reaction product A was analyzed by high performance liquid phase to obtain the mass percentage of each component. Weigh 40.0g of methanol and add it to the above reaction product A and mix well, then add 200g of diethyl phthalate, seal the reaction device, and pass nitrogen gas with a flow rate of 600ml / min into it, and control the pressure of the reaction device It is 4MPa and heated to 200°C. Stay at 200°C for 20 minutes, stop heating, and cool down naturally to room temperature. Finally, open the device and collect 408.9g of reaction product B. Perform high-performance liquid phase analysis on reaction product B to obtain the content of each component in the product, including monophenylurea Undetectable, diphenylurea accounts for 0.23%, methyl phenylcarbamate accounts for 29.21%, anili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com