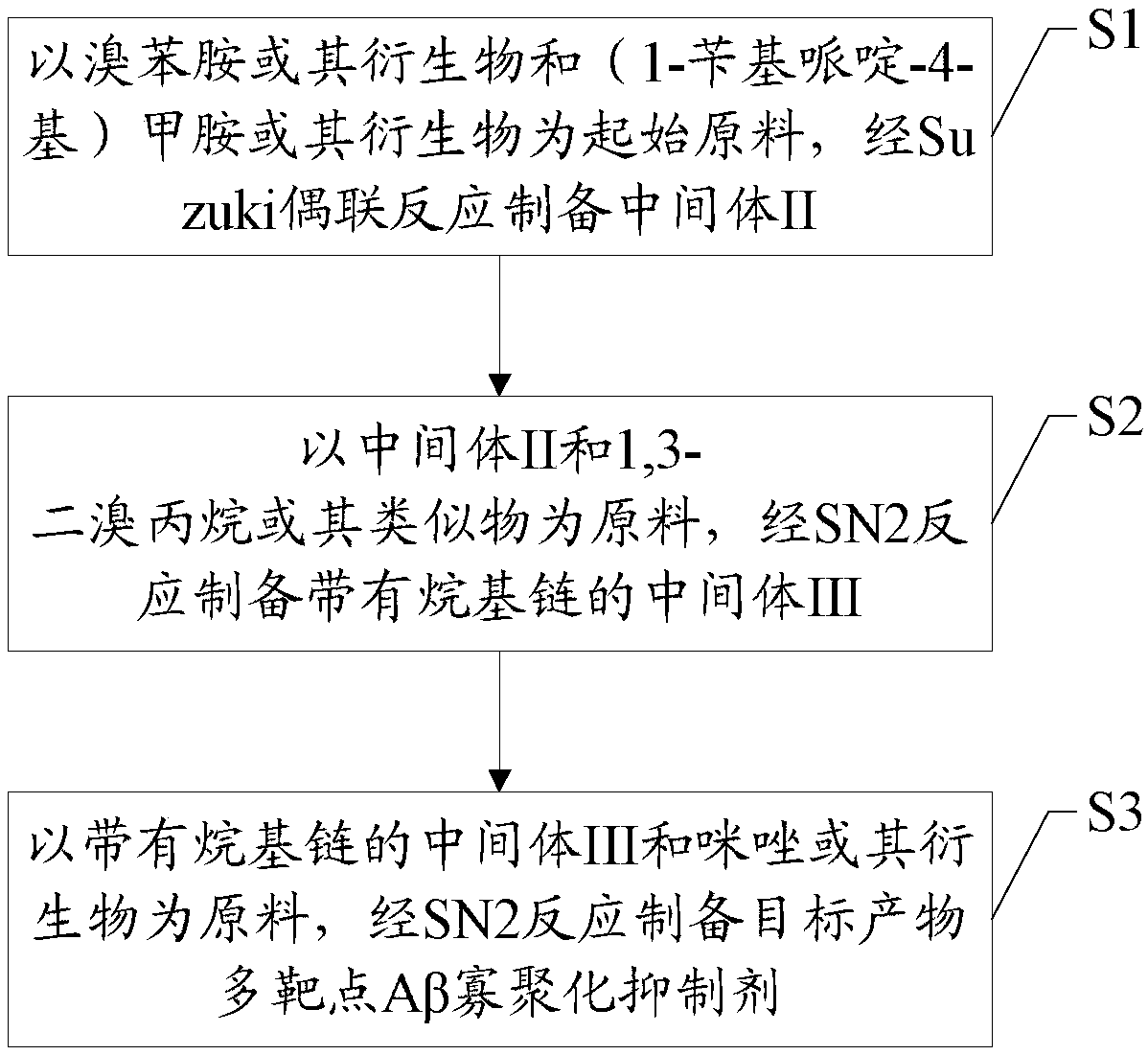
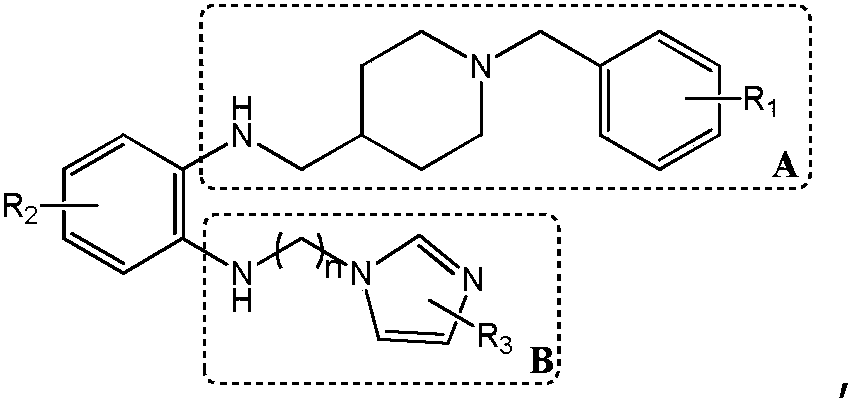
Synthesis method and application of a multi-target Aβ oligomerization inhibitor
A synthesis method and inhibitor technology, applied in organic chemistry, drug combination, nervous system diseases, etc., can solve the problems of product performance to be improved, complex and cumbersome synthesis method, etc., and achieve the effect of simple and reliable route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of N1-(3-(1H-imidazol-1-yl) propyl)-N2-((1-benzyl-4-yl)methyl)benzene-1,2-diamine, which The synthetic route is as follows:
[0038]
[0039]
[0040] a, 3N1-((1-benzyl-4-yl)methyl)benzene-1,2-diamine (II) synthesis: bromoaniline (5.81mmol, 1equiv), (1-benzylpiperidine- 4-yl)methylamine (6.98mmol, 1.2equiv) was dissolved in dioxane (10ml) and 2mol / L potassium carbonate (10ml) mixed solution, adding catalyst [1,1'-bis(diphenylphosphine) Ferrocene] dichloropalladium (II) dichloromethane complex (0.3486mmol, 0.06equiv); ventilation, so that the whole device is filled with argon; the mixed system was refluxed at 100°C for 3h, extracted 3 times with ethyl acetate , combined organic phase, washed once with saturated NaCl solution, anhydrous NaCl 2 SO 4 After drying, the product was collected by silica gel column chromatography with a yield of 90%.
[0041]b. Synthesis of N1-((1-benzyl-4-yl)methyl)-N2-(3-bromopropyl)benzene-1,2-diamine (Ⅲ): 3N1-(...
Embodiment 2
[0043] Example 2: Synthesis of N2-(3-(1H-imidazol-1-yl) propyl)-N3-((1-benzyl-4-yl)methyl)naphthalene-2,3-diamine, which The synthetic route is as follows:
[0044]
[0045] a, N2-((1-benzyl-4-yl)methyl)naphthalene-2,3-diamine (II) synthesis: 3-bromo-2-naphthylamine (5.81mmol, lequiv), (1 -benzylpiperidin-4-yl)methanamine (6.98mmol, 1.2equiv) was dissolved in dioxane (15ml) and 2mol / L potassium carbonate (10ml) mixed solution, adding catalyst [1,1'-bis (Diphenylphosphine) ferrocene] dichloropalladium (II) dichloromethane complex (0.3486mmol, 0.06equiv); ventilation, so that the whole device is filled with argon; the mixed system was refluxed at 100°C for 4h, Extracted three times with ethyl acetate, combined organic phase, washed once with saturated NaCl solution, anhydrous NaCl 2 SO 4 After drying, the product was collected by silica gel column chromatography with a yield of 88%.
[0046] B, the synthesis of N2-((1-benzyl-4-yl)methyl)-N3-(3-bromopropyl)naphthalene-2,3-...
Embodiment 3
[0048] Example 3: N1-(3-(1H-imidazol-1-yl)propyl)-N2-((1-benzyl-4-yl)methyl)benzene-1,2-diamine on Aβ oligomerization Chemical inhibition:
[0049] Such as figure 2 As shown, it is N1-(3-(1H-imidazol-1-yl)propyl)-N2-((1-benzyl-4-yl)methyl)benzene-1,2-diamine to Aβ42 oligo Comparison diagram of polymerization inhibition, in which, 1 is the protein marker; 2-3 is the control group; 4-5 is the sample addition group.
[0050] Prepare the DMSO monomer stock solution (1mM) of Aβ42 immediately before use, and prepare N1-(3-(1H-imidazol-1-yl)propyl)-N2-((1-benzyl-4-yl)methyl)benzene -1,2-diamine in DMSO stock solution (1 mM), take 4 μL Aβ42 stock solution and 4 μL N1-(3-(1H-imidazol-1-yl)propyl)-N2-((1-benzyl-4-yl )Methyl)benzene-1,2-diamine mother solution was mixed, 2 μL of 1% SDS solution was added, the total volume was adjusted to 15 μL with PBS buffer solution, incubated at 37°C for 6 and 12 hours, and SDS-PAGE analysis was carried out after sampling (15 % separating gel). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com