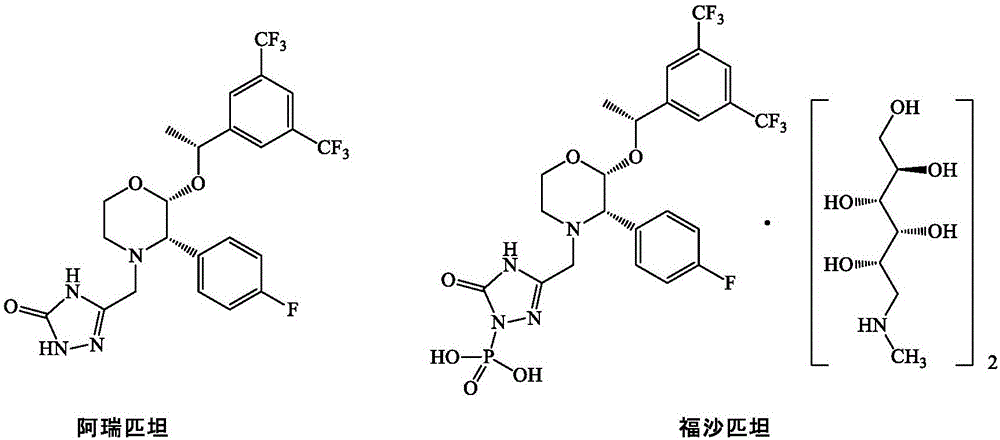
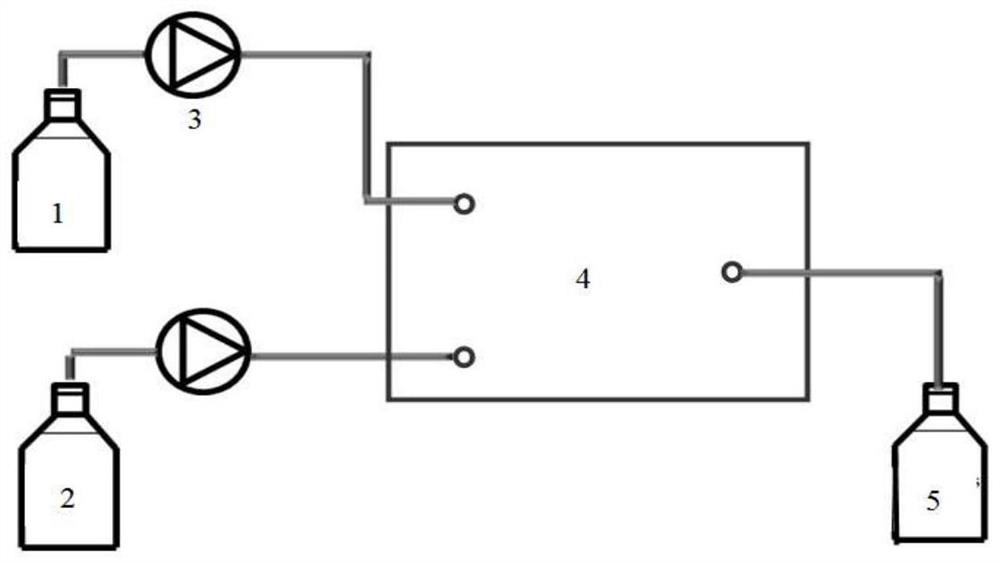
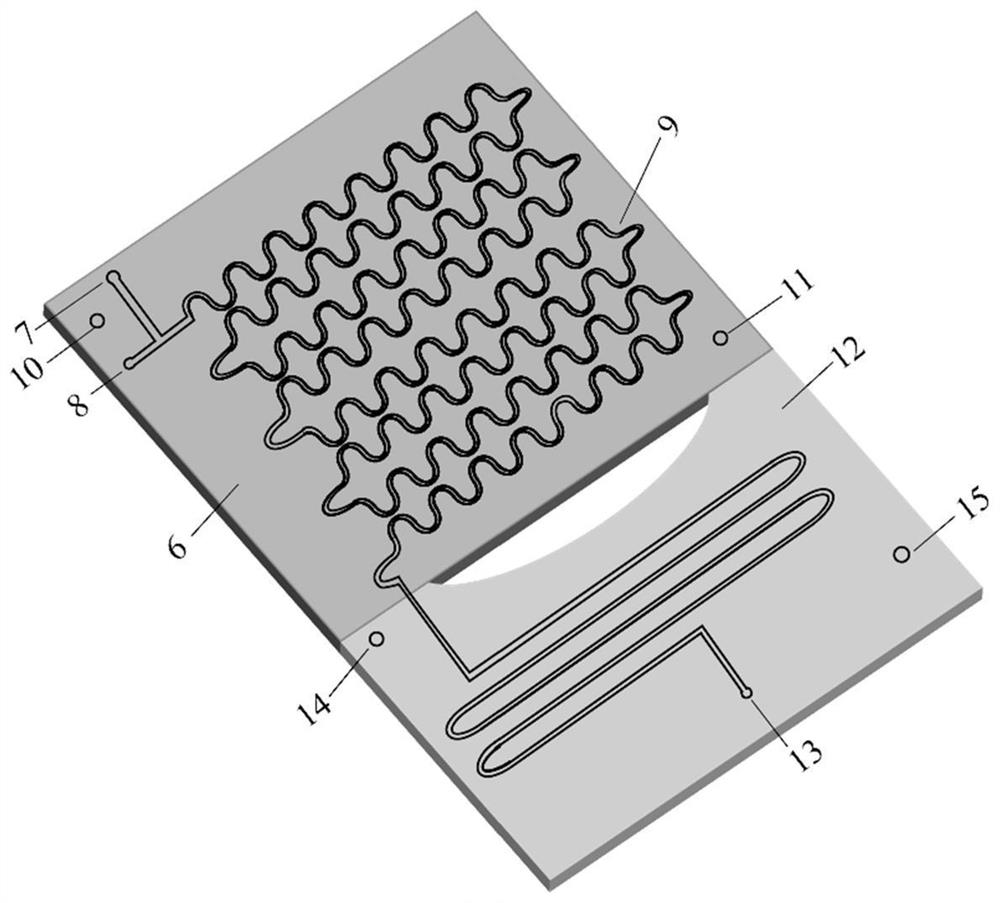
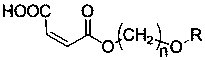
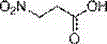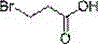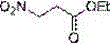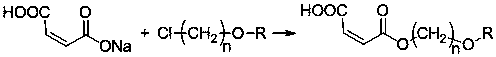Patents
Literature
34 results about "SN2 reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed synchronously, i.e., in one step. SN2 is a kind of nucleophilic substitution reaction mechanism. Since two reacting species are involved in the slow (rate-determining) step, this leads to the term substitution nucleophilic (bi-molecular) or SN2, the other major kind is SN1. Many other more specialized mechanisms describe substitution reactions.
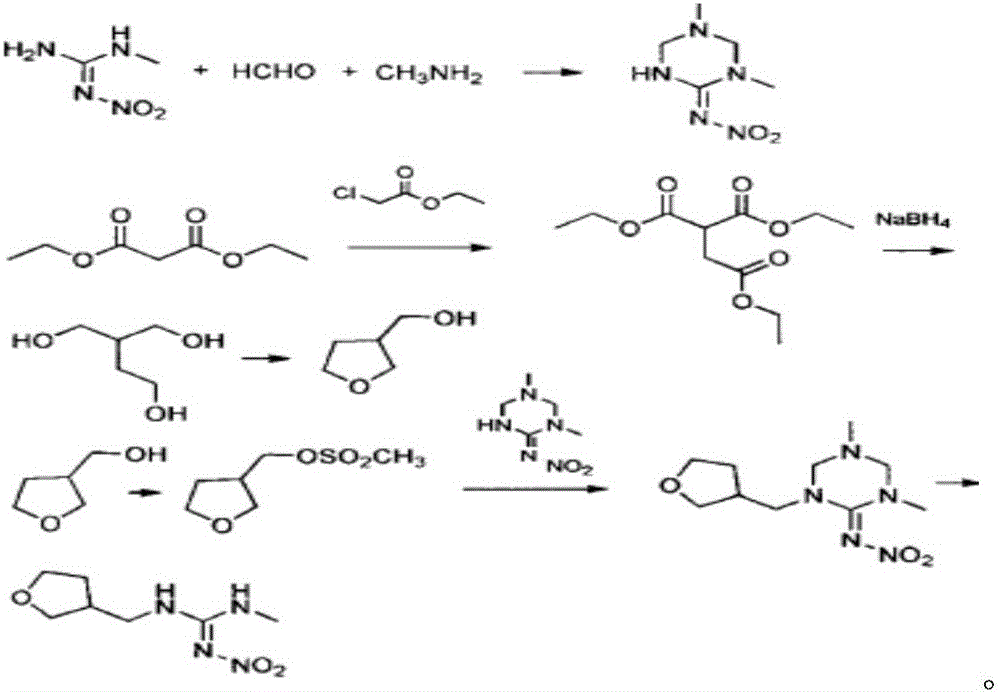
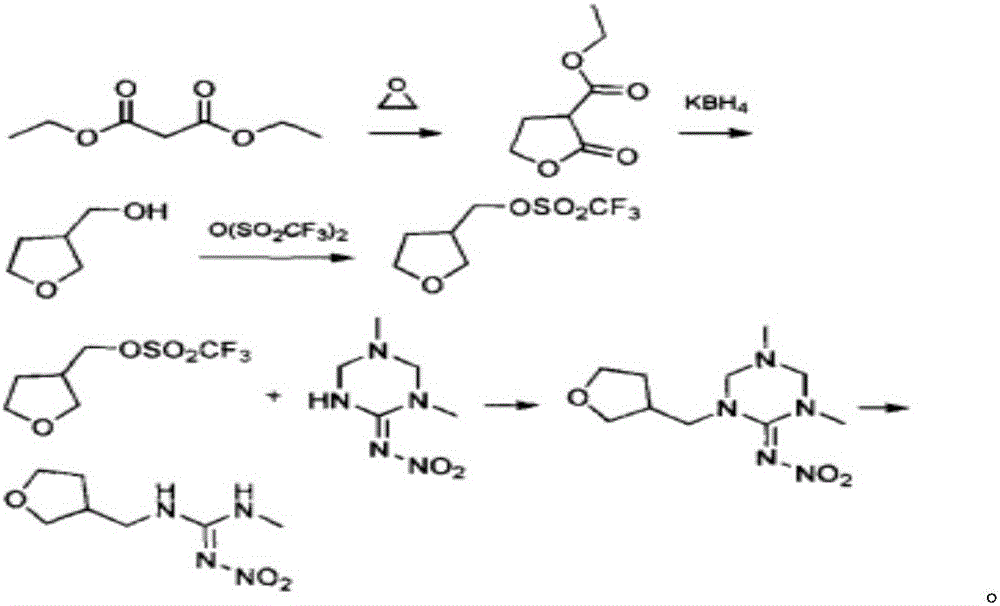
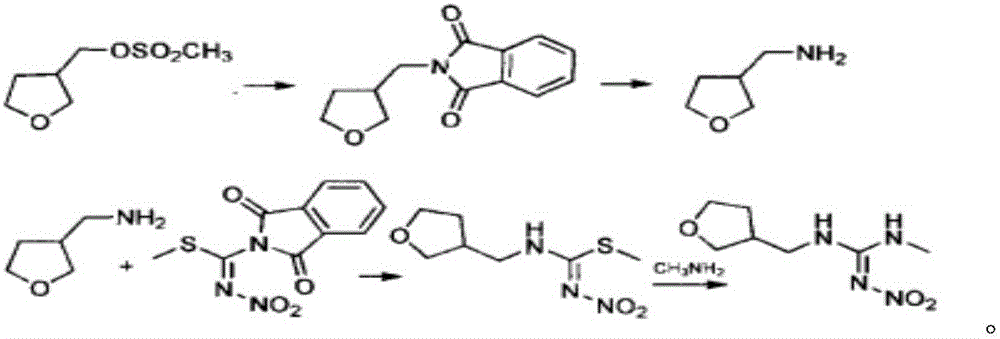
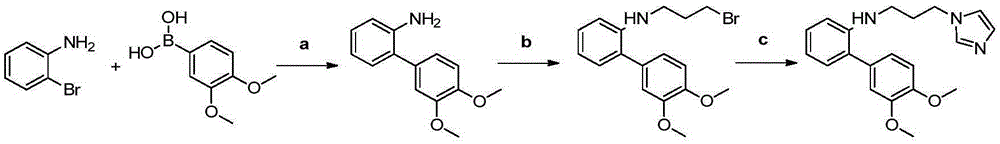
Synthesis method of dinotefuran
ActiveCN106349196AAvoid pollutionAvoid production operation hazardsUrea derivatives preparationOrganic compound preparationCarbylamine reactionMethyl carbonate
The invention discloses a synthesis method of dinotefuran. The synthesis method includes: using gamma-butyrolactone as a raw material, and preparing 3-hydroxymethyltetrahydrofuran through two-step reaction of Aldol condensation and reduction; synthesizing 3-tetrahydrofuran methylamine through a Gabriel method; using dimethyl carbonate (DMC) as a methylation reagent to synthesize with urea to obtain O-methylisourea; performing nitration to synthesize O-methyl-N-nitroisourea; reacting with methylamine to synthesize 1, 3-dimethyl-2-nitroisourea; subjecting 3-tetrahydrofuran methylamine and 1, 3-dimethyl-2-nitroisourea to a one-step process to synthesize dinotefuran through SN2dimolecular nucleophilic substitution. Dimethyl carbonate which is nontoxic is adopted as the methylation agent, methylation reaction yield is greater than or equal to 95%, O-methylisourea content is greater than or equal to 96%, byproducts are carbon dioxide and methanol, the process is environment-friendly, and total yield of dinotefuran is greater than or equal to 50%. The synthesis method can lower production cost, has the advantages of little three wastes, simple aftertreatment and environment friendliness, is suitable for industrial production and can bring about remarkable economic benefit.
Owner:JIANGSU KESHENG CROP TECH
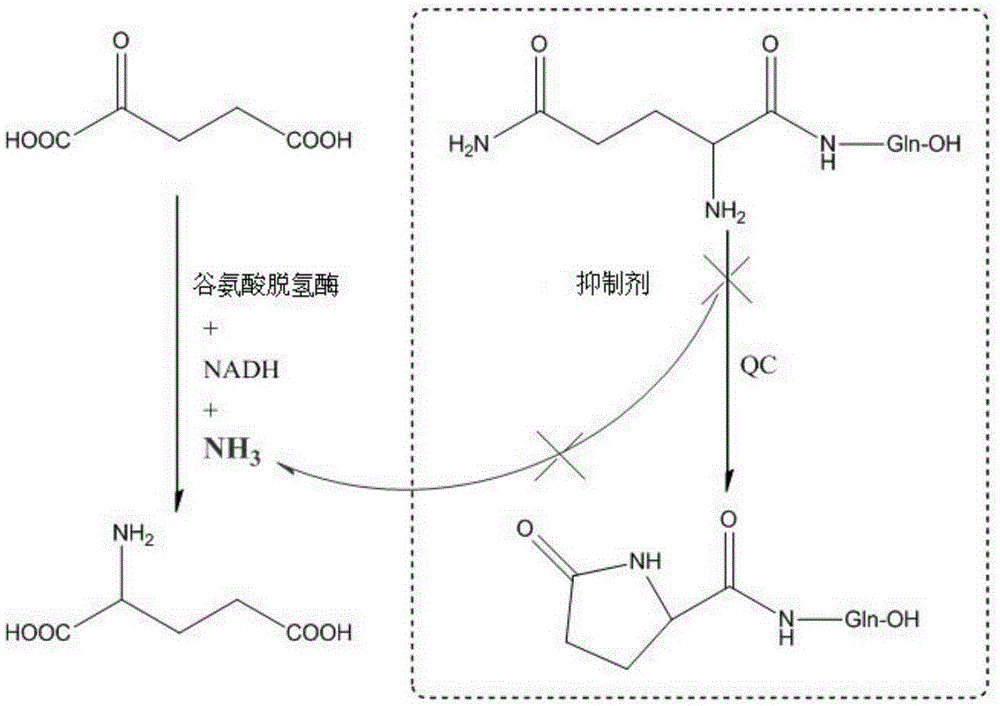
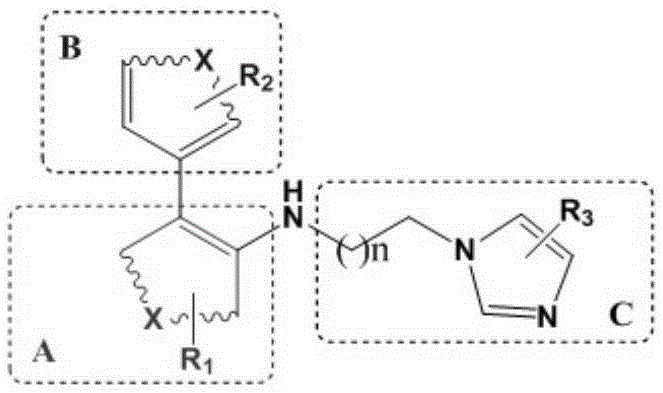
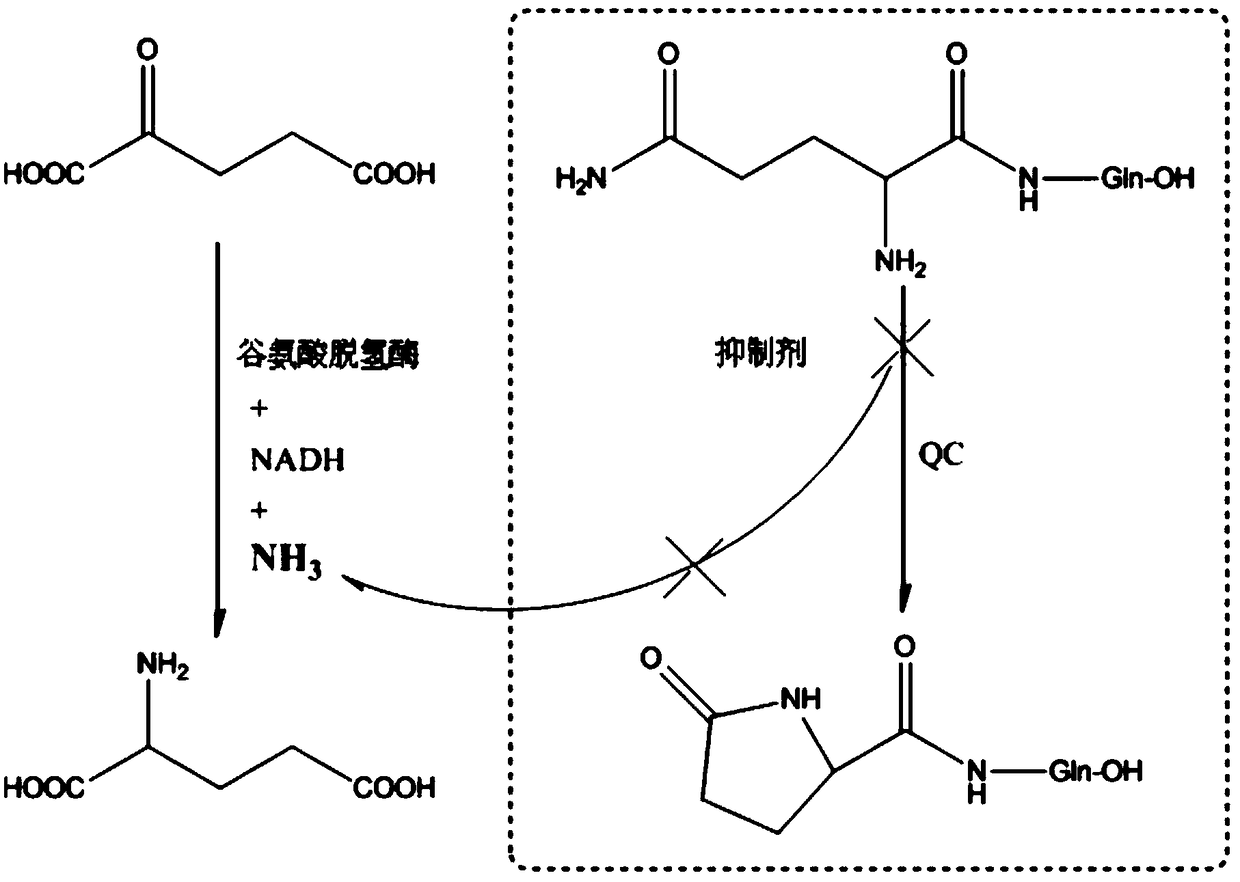
Preparation method and application of glutaminylcyclase (QC) inhibitor
ActiveCN105384691ASimple process routeHigh yieldOrganic active ingredientsNervous disorderDiseaseBromine
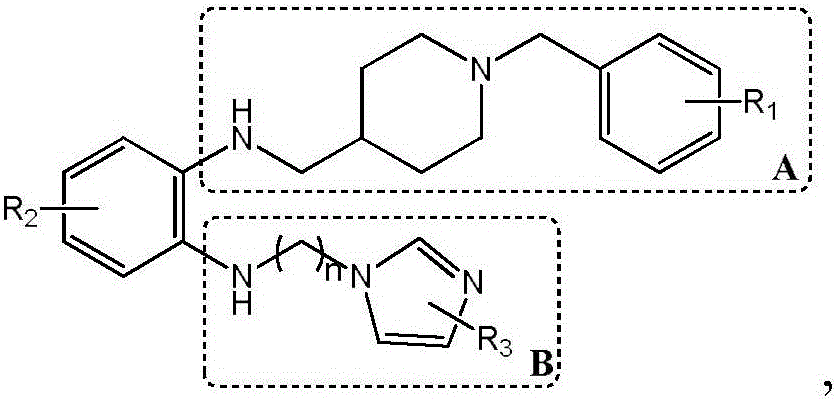
The invention discloses a preparation method and application of a glutaminylcyclase (QC) inhibitor. The preparation method comprises the of following steps that (1) raw materials of a unit A including a borate group, amino and R1 and raw materials of a unit B including a bromine substituent group and R2 serving as starting materials are subjected to a Suzuki coupling reaction to prepare a unit A and unit B coupled intermediate; (2) the unit A and unit B coupled intermediate and a dibromoalkyl chain serving as raw materials are subjected to an SN2 reaction to prepare an intermediate containing an alkyl chain; and (3) the intermediate containing the alkyl chain and imidazole or substituent-containing imidazole serving as raw materials are subjected to the SN2 reaction to prepare the QC inhibitor. The preparation method can be completed only by means of Suzuki coupling, SN2 reaction and the like, is simple and feasible in process route and high in yield, and is more suitable for large-scale preparation; and the QC inhibitor can be widely applied to development of new high-efficient QC inhibitor drugs, development of drugs, innovative drugs and diagnostic kits for alzheimer disease (AD) and other QC specifically highly expressed related diseases, and the like.
Owner:SHENZHEN UNIV
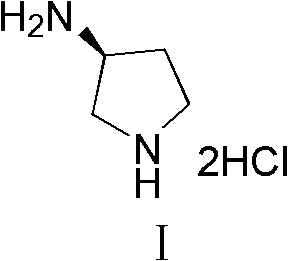
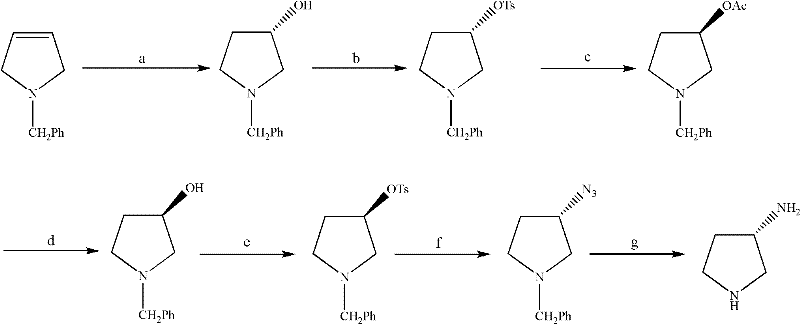
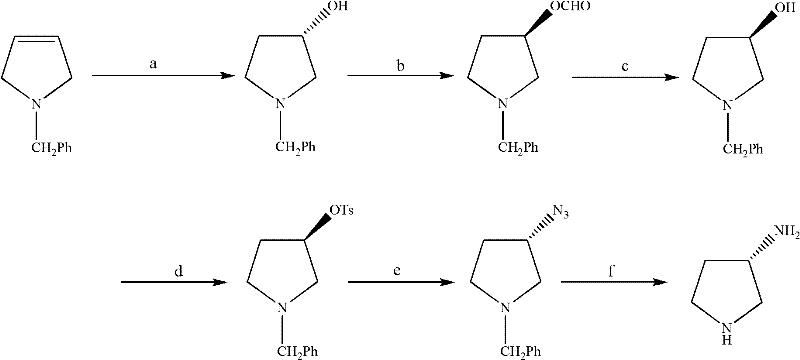
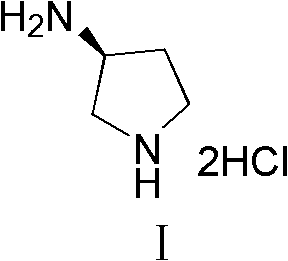
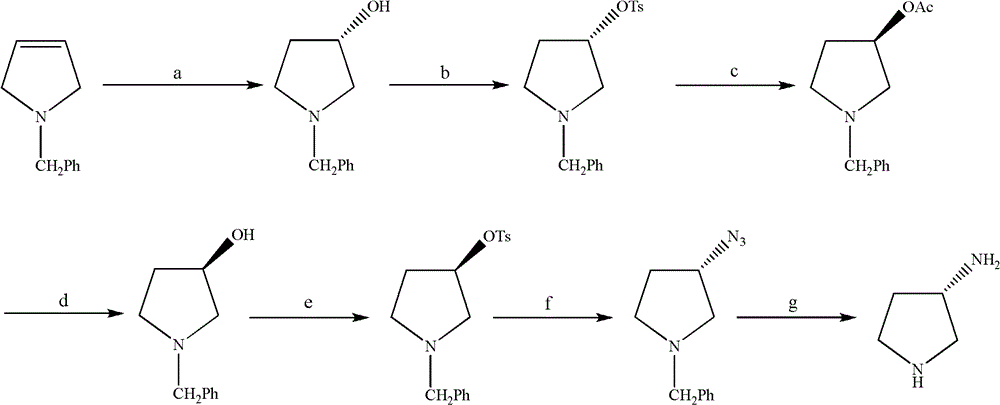
Synthesis method of (S)-3-amino pyrrolidine dihydrochloride
ActiveCN102531987ARaw materials are cheap and easy to getLow costOrganic chemistryTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention relates to a chiral synthesis method of (S)-3-amino pyrrolidine dihydrochloride. Trans-4-hydroxyl-L-proline is taken as raw materials, is first subjected to decarboxylation and then subjected to N-tert-butoxycarbonyl (N-Boc) protection and hydroxyl sulfonylurea acylation, and is then subjected to an SN2 reaction with sodium azide and configuration reversal. After azido is changed to amino through reduction by triphenylphosphine, concentrated hydrochloric acid is directly used for removal of N-Boc protection, and target products are obtained through a total of four steps of chain reactions. Raw materials of the chiral synthesis method are easy to obtain, reaction conditions are mild, post-processing is simple and convenient, and product yield is high.
Owner:SHANGHAI INST OF PHARMA IND +1
Preparation method of baloxavir intermediate
ActiveCN111018803AEasy to respondMild conditionsOrganic chemistry methodsBulk chemical productionHydroxylaminePtru catalyst
Owner:苏州楚凯药业有限公司
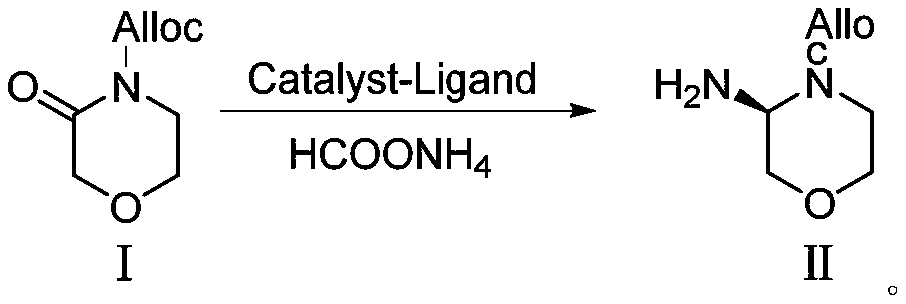
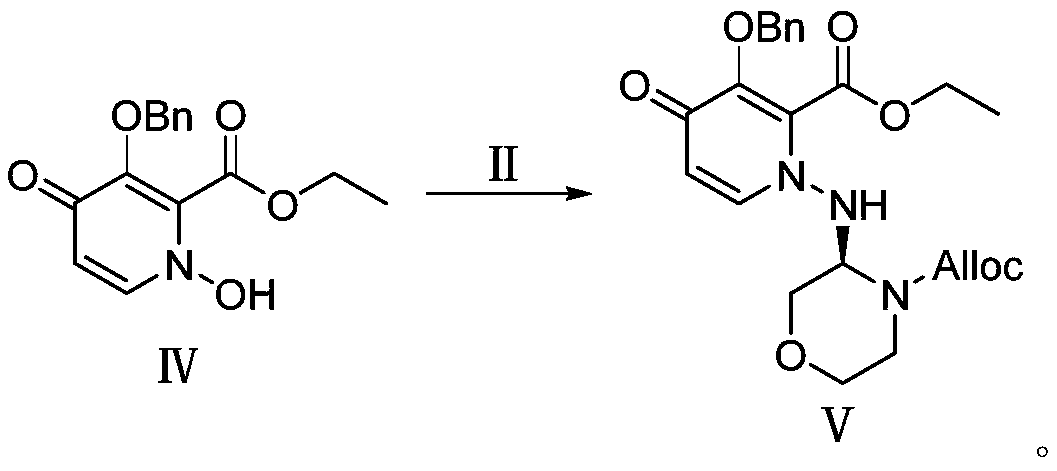
Preparation method of morpholine derivative
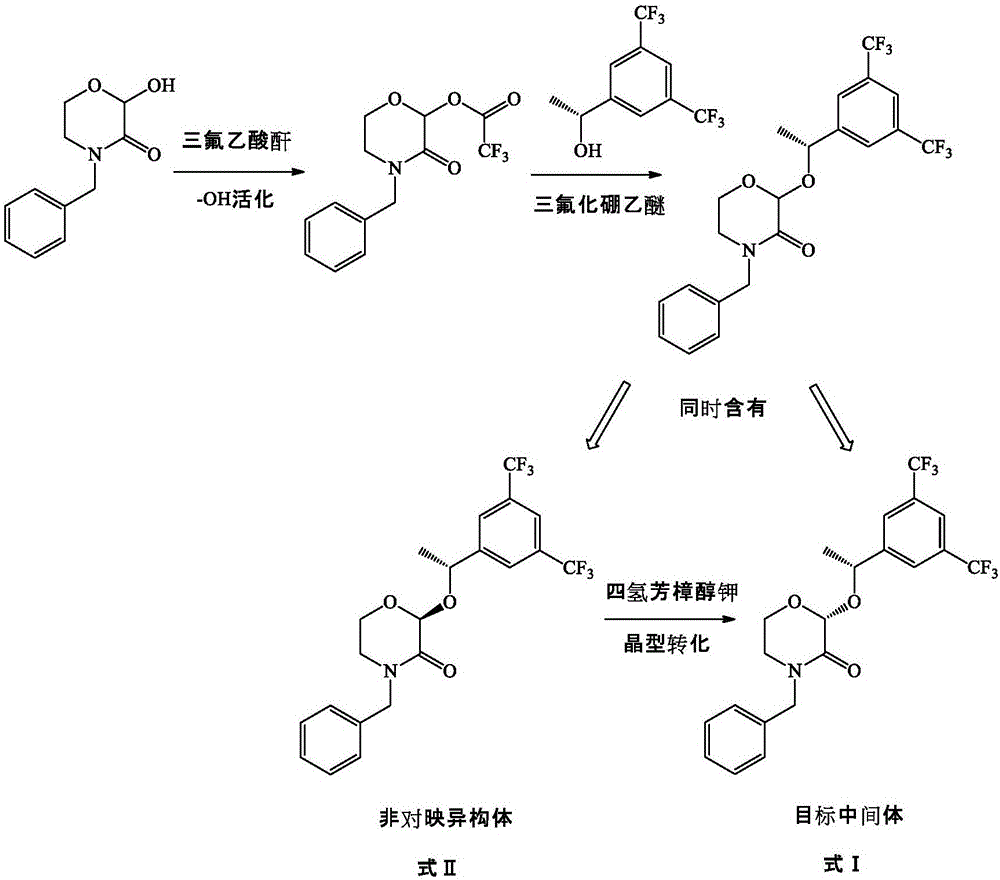
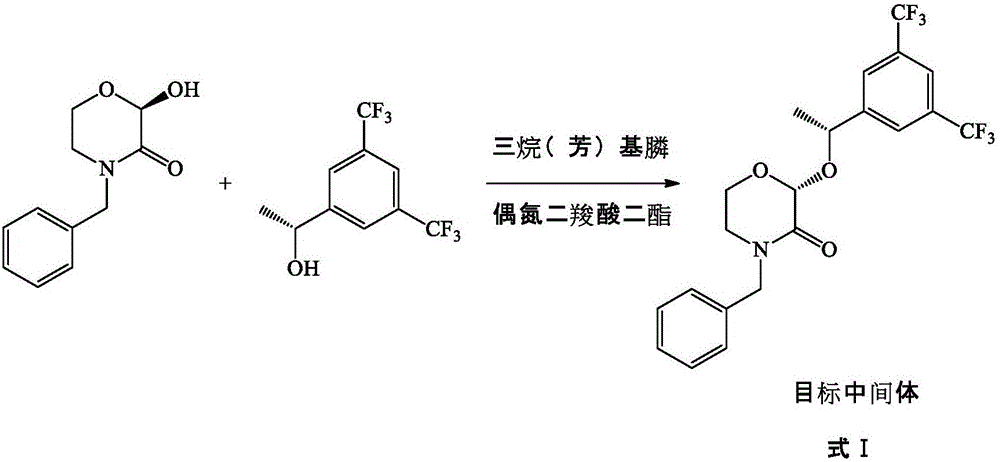
The invention provides a preparation method of a morpholine derivative represented as the formula (I), wherein the preparation method includes the steps of: (1) dissolving trialkyl (aryl) phosphine, azodicarboxylic acid diester, (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethyl-1-ol, and (R)-4-benzyl-2-hydroxyl-morpholine-3-one in a reaction solvent; (2) adding the azodicarboxylic acid diester so that the trialkyl (aryl) phosphine and the azodicarboxylic acid diester are subjected to an addition reaction quickly in the reaction solvent to generate zwitter-ions, which are then converted into quaternary phosphonium salt by means of a hydrogen proton supplied by the hydroxyl group in the (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethyl-1-ol; (3) enabling the hydroxyl group on the 2-position of the (R)-4-benzyl-2-hydroxyl-morpholine-3-one to be reacted with the quaternary phosphonium salt to generate morpholine oxy-phosphonium salt; and (4) with the (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethyl-1-ol, which loses the hydrogen proton, as a nucleophilic reagent, performing an SN2 reaction with the morpholine oxy-phosphonium salt to obtain a product which has turned configuration. The method has high stereo-selectivity, avoids generation of an isomer byproduct during the reaction process, and reduces the load of separation and purification of the product in the later period.
Owner:江苏富泽药业有限公司
Method for producing high-viscosity and high-degree-of-substitution carboxymethyl cassava starch by utilizing ultrahigh-pressure blasting technique
The invention discloses a method for producing high-viscosity and high-degree-of-substitution carboxymethyl cassava starch by utilizing an ultrahigh-pressure blasting technique. The method comprises the steps such as alkaline treatment, etherification reaction and purification. The method is carried out by primarily achieving the purpose of destroying a crystallization region of cassava starch particles by utilizing sodium hydroxide, sufficiently swelling and alkalifying starch molecules and loosening starch particles under the ultrahigh pressure of a high-pressure homogenizer, so that the hydroxyl of the starch becomes negative oxygen ions and the nucleophilic ability is strengthened, and simultaneously generating an St-O-Na<+> active center, then, preparing the high-viscosity and high-degree-of-substitution carboxymethyl cassava starch through the bimolecular nucleophilic substitution between sodium carboxymethyl starch obtained by alkalifying the starch and chloroacetic acid under the alkaline condition of sodium hydroxide. By utilizing the high-degree destructive effect of the ultrahigh-pressure blasting technique on a crystallization structure of the cassava starch, the technical problems such as degradation of the starch particles in a production process of the high-viscosity and high-degree-of-substitution carboxymethyl cassava starch are solved.
Owner:GUANGXI JINHAN ENVIRONMENTAL PROTECTION CO LTD
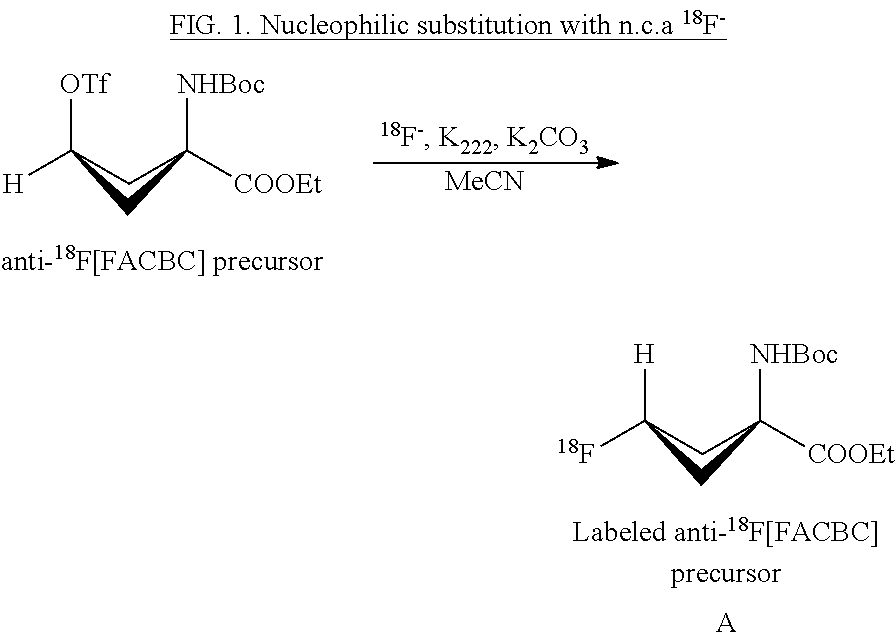
Borosilicate glassware and silica based QMA's in 18f nucleophilic substitution: influence of aluminum, boron and silicon on the reactivity of the 18f- ion
InactiveUS20120229804A1Emission spectroscopyRadiation pyrometrySN2 reactionNucleophilic substitution
Aluminum, boron and silicon were identified as potential leachables from borosilicate glassware and a silica based QMA during handling of aqueous 18F. The addition of only 0.4 ppm aluminum as AlCl3 in the eluent vial resulted in a strong reduction in the labeling yield of a model [18F]fluoride SN2 reaction (from 80 to 40% incorporation). The addition of boron as KBO2 and silicon as NaSiO3 did not result in any significant decrease in labeling yield. Interestingly, there was an interaction effect between AlCl3 and KBO2 in which the negative effect from AlCl3 on labeling yield was counteracted by KBO2. The present invention demonstrates that aluminum and boron from borosilicate glassware have a strong influence on the labeling yield in nucleophilic SN2 reactions with n.c.a [18F]fluoride.
Owner:GE HEALTHCARE LTD
Kras-G12C inhibitor heterocyclic compounds
ActiveCN112574199AHigh activityImprove mechanical propertiesOrganic active ingredientsOrganic chemistry methodsDiseasePancreas Cancers
The invention relates to Kras-G12C inhibitor heterocyclic compounds and a preparation method thereof, and application of the compounds in prevention and treatment of tumor diseases such as lung cancer, colorectal cancer and pancreatic cancer. In the preparation process, the compounds provided by the invention are obtained through a series of reactions such as a condensation reaction, an intramolecular cyclization reaction, a chlorination reaction, an SN2 reaction, a coupling reaction, deprotection and the like.
Owner:SHOUYAO HLDG BEIJING CO LTD
Preparation method and intermediate of lepimectin
ActiveCN111217828AReduce manufacturing costHigh yieldOrganic chemistryBulk chemical productionBiochemical engineeringAcid hydrolysis
The invention discloses a preparation method and an intermediate of lepimectin represented by a formula V. The preparation method comprises the following steps: by taking tenvermectin as an initial raw material, carrying out an acid hydrolysis reaction, adding a protecting group to a 5-hydroxyl group, then carrying out an SN2 reaction with 2-methoxyiminobenzeneacetic acid, and finally removing the5-hydroxyl protecting group to obtain the compound represented by the formula V. The preparation method has the advantages of low cost, high purity, high yield and suitability for industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
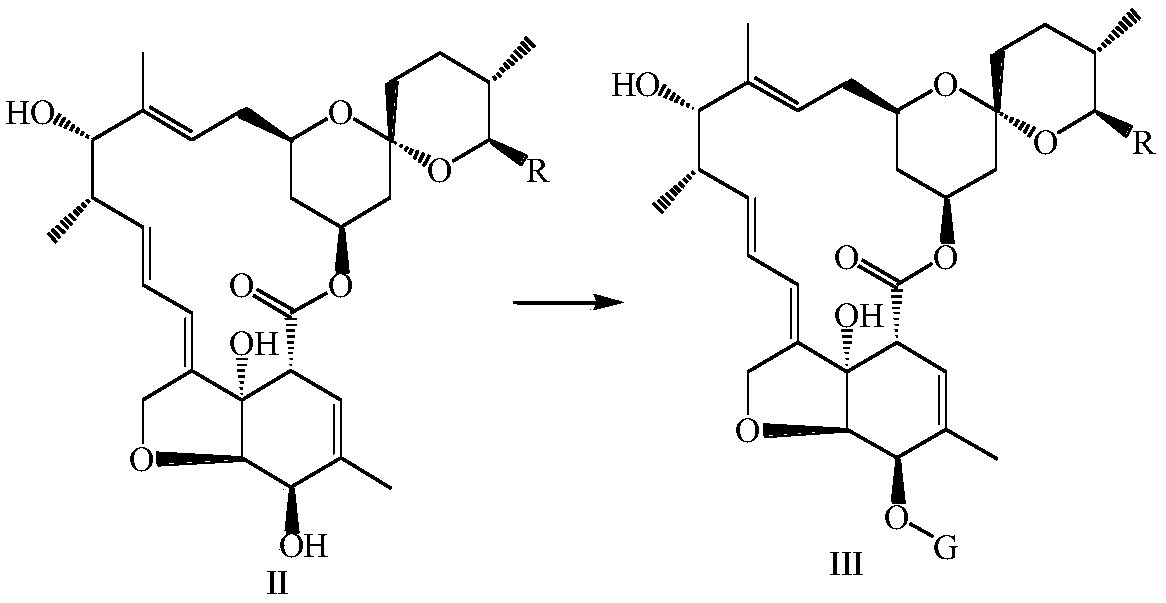
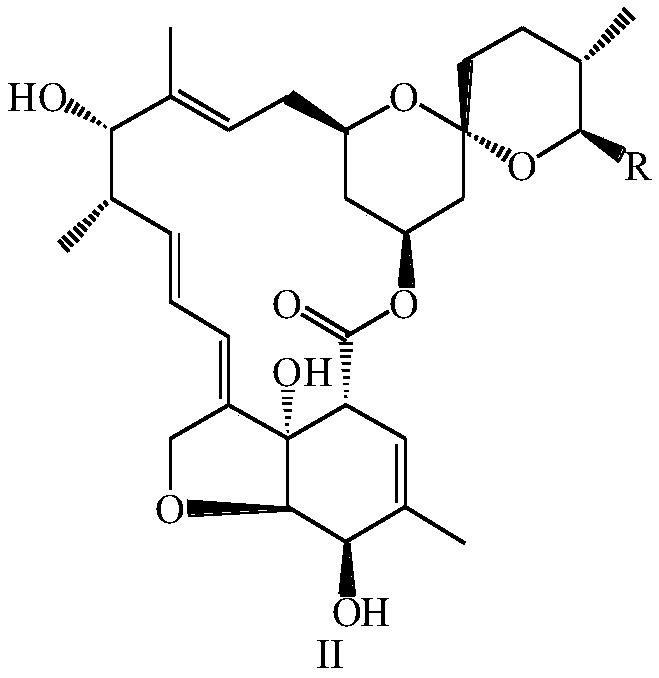
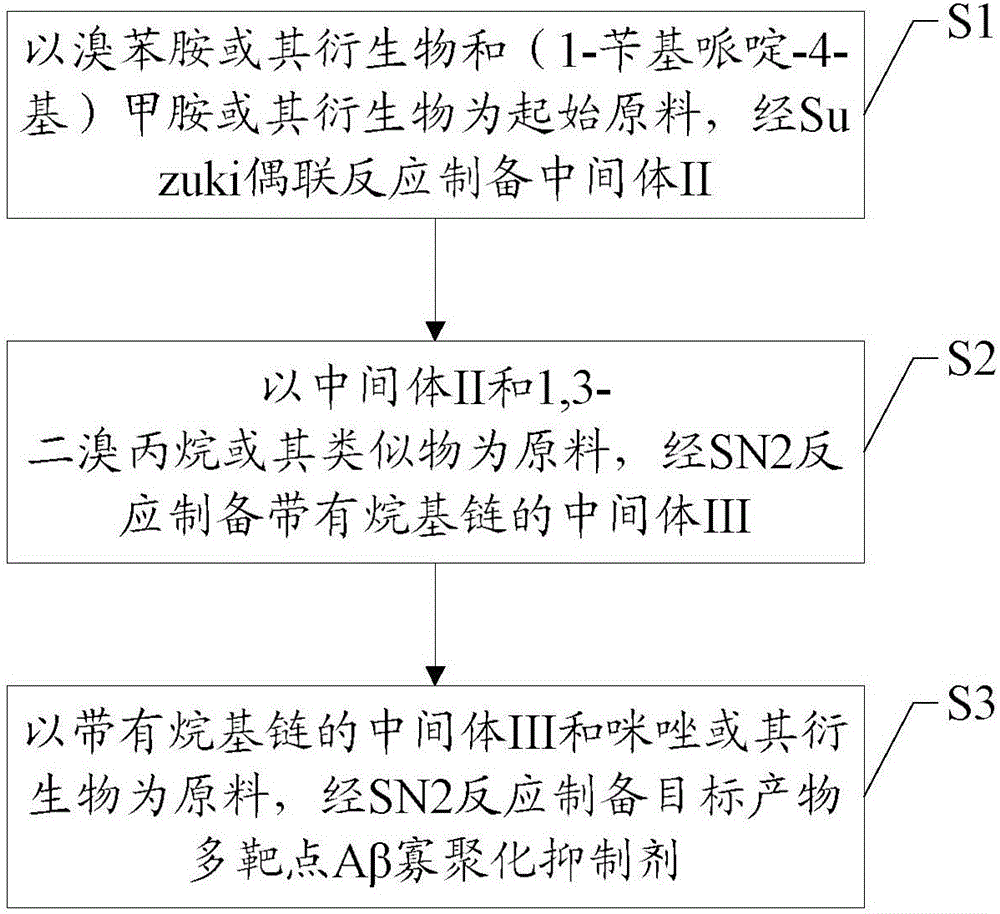
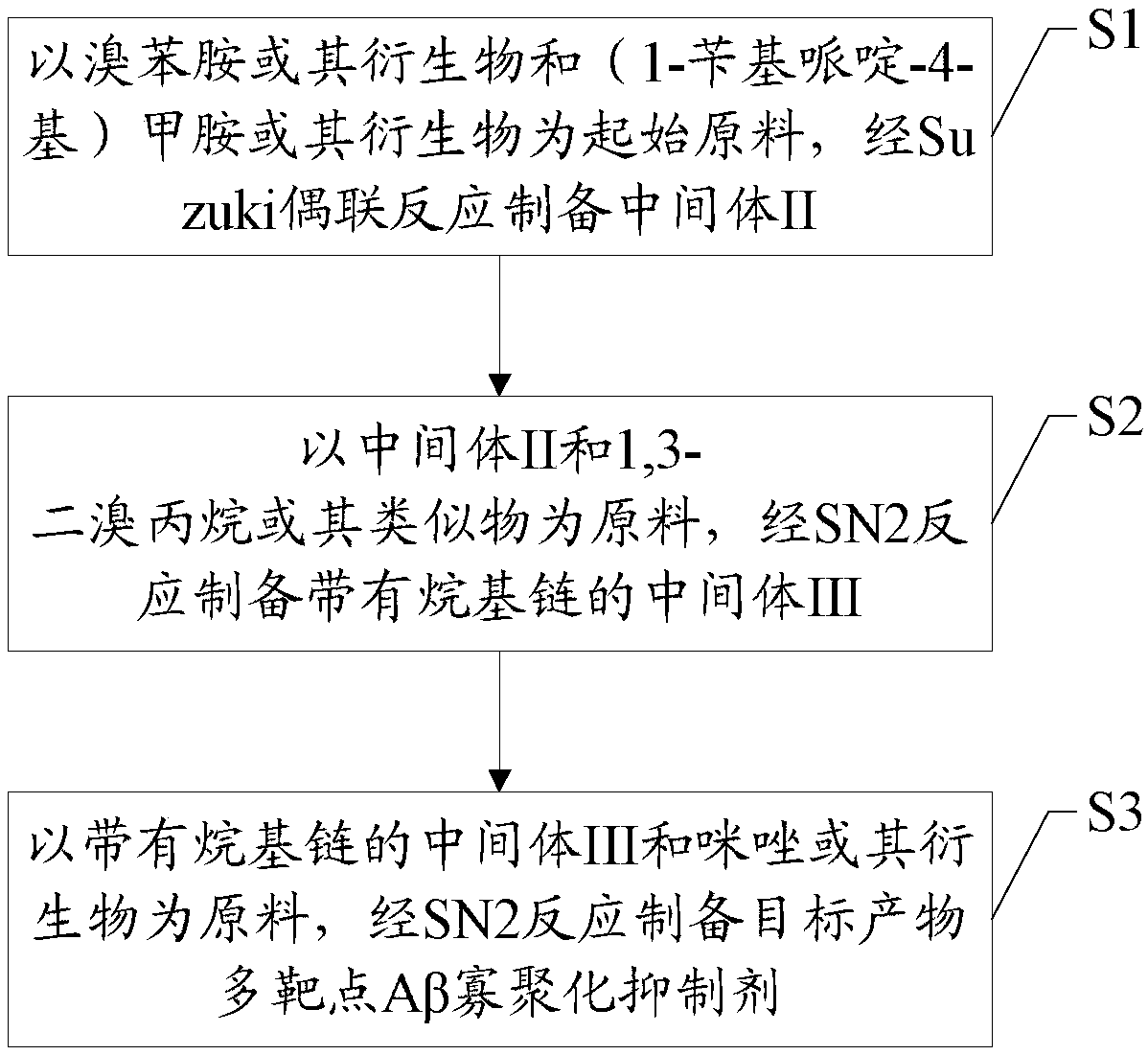
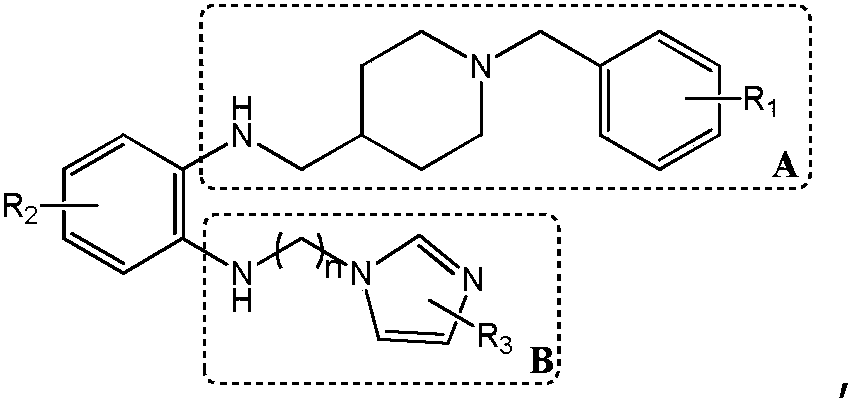
Synthesis method and application of multi-target A beta oligomerization inhibitor
ActiveCN106831713AHigh yieldSuitable for mass manufacturingNervous disorderOrganic chemistrySynthesis methodsDibromopropane
The invention discloses a synthesis method and application of a multi-target A beta oligomerization inhibitor. The synthesis method comprises the following steps: (1) taking bromaniline or derivatives thereof and (1-benzylpiperidine-4-yl)methylamine or derivatives thereof as starting raw materials, and carrying out Suzuki coupling reaction for preparing an intermediate II; (2) taking the intermediate II and 1,3-dibromopropane or other analogues as raw materials, and carrying out SN2 reaction for preparing an intermediate III with an alkyl chain; and (3) taking the intermediate III with the alkyl chain and imidazole or derivatives thereof as raw materials, and carrying out SN2 reaction for preparing the target product multi-target A beta oligomerization inhibitor. The synthesis method disclosed by the invention is simple and reliable in route, high in yield and more suitable for large-scale preparation. The multi-target A beta oligomerization inhibitor is applied to preparation of an A beta oligomerization inhibitor anti-AD medicine, a glutaminyl cyclase inhibitor anti-AD medicine and an acetylcholin esterase inhibitor anti-AD medicine and can be applied to kits of A beta oligomerization, glutaminyl cyclase and acetylcholin esterase.
Owner:SHENZHEN UNIV
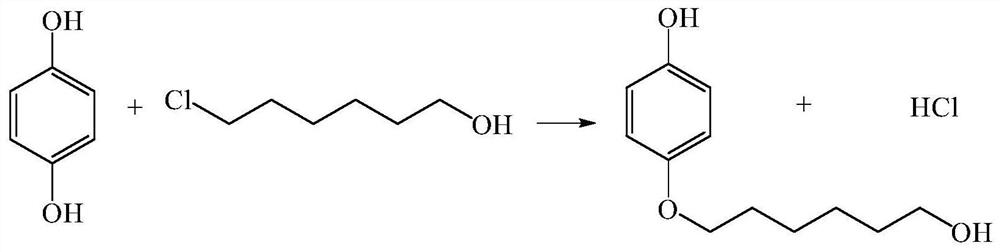
Method for continuously preparing 4-(6-hydroxyhexyloxy)phenol
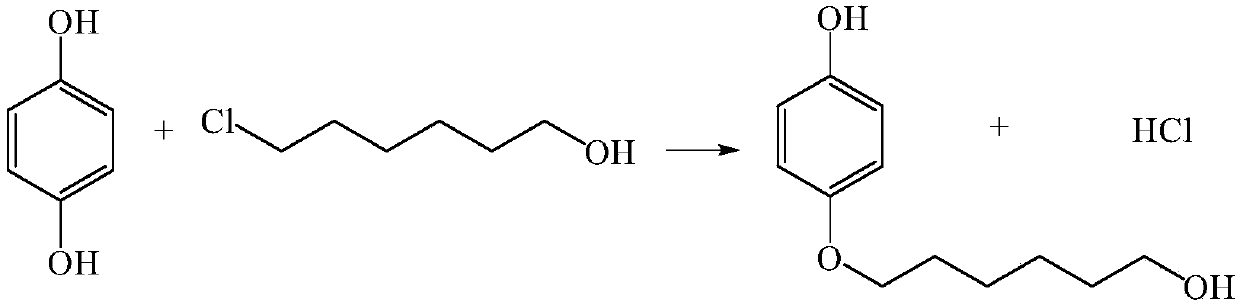
ActiveCN107867979AHigh yieldContinuous operationEther separation/purificationChemical/physical/physico-chemical microreactorsSN2 reactionHydroquinone Compound
The invention discloses a method for continuously preparing 4-(6-hydroxyhexyloxy)phenol in a micro-reactor. The method comprises the following steps: dissoling hydroquinone and 6-chloro-1-hexanol in the solvent ethanol; and with an alkali aqueous solution of NaOH<-> as a catalyst, carrying out a bimolecular nucleophilic substitution reaction in the micro-reactor so as to continuously prepare 4-(6-hydroxyhexyloxy)phenol. Compared with the prior art, the method provided by the invention has the following advantages that the synthesis time of 4-(6-hydroxyhexyloxy)phenol is reduced to 10 min or less from original 20 hours; yield is increased from original 40% to 80%;continuous operation during the reaction can be realized; and integration and enlargement can be easily realized.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing 3-nitropropionic acid
InactiveCN104447346AEasy to operateMild conditionsOrganic chemistryOrganic compound preparationPropanoic acidSN2 reaction
The invention discloses a method for preparing 3-nitropropionic acid. Acrylic acid serves as a raw material, and the 3-nitropropionic acid is prepared by the following four steps: performing olefin addition, performing esterification, carrying out an SN2 reaction and hydrolyzing. Compared with the other methods, the method has the advantages of readily available raw materials, low price, simplicity in operation, mild and controllable conditions, high yield and the like.
Owner:QUFU DELU BIOTECH
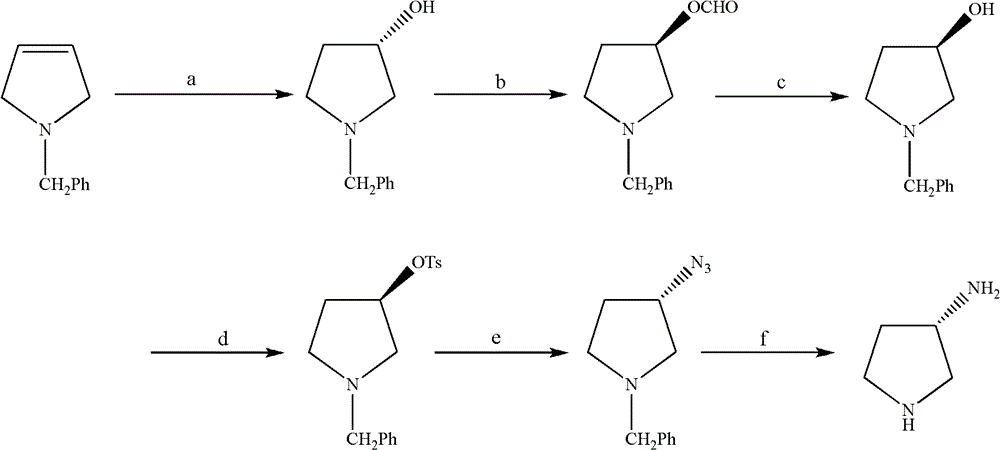
The synthetic method of (s)-3-aminopyrrolidine dihydrochloride
ActiveCN102531987BRaw materials are cheap and easy to getLow costOrganic chemistrySynthesis methodsTert-Butyloxycarbonyl protecting group
The invention relates to a chiral synthesis method of (S)-3-aminopyrrolidine dihydrochloride, which uses trans-4-hydroxyl-L-proline as a raw material, first undergoes a decarboxylation reaction, and then performs N-tert-butyl Oxycarbonyl protection, hydroxyl sulfonylation, and then SN2 reaction with sodium azide, configuration inversion, after triphenylphosphine reduction of azido group to amino group, directly remove N-Boc protection with concentrated hydrochloric acid, a total of A four-step chain reaction yields the target product. The raw materials used in the inventive method are cheap and easy to obtain, the reaction conditions are mild, the aftertreatment is simple and convenient, and the yield of the obtained product is relatively high.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Concrete release agent prepared from chloroacetic acid modified konjac glucomannan (KGM)
InactiveCN106493839ANo pollution in the processEasy to useCeramic shaping apparatusLubricant compositionWater basedSN2 reaction
The invention discloses a concrete release agent prepared from chloroacetic acid modified konjac glucomannan (KGM). KGM and chloroacetic acid have a dimolecular nucleophilic substitution reaction in a sodium hydroxide alkaline environment. A preparation method of the concrete release agent includes the steps that KGM purified powder is placed into a beaker containing 60-75% of an alcoholic solution, chloroacetic acid is added, the pH value is adjusted to 8-9, stirring is conducted at 60 DEG C for a reaction for 3-4h, and modified KGM particles are obtained after drying; and water is added for stirring and dissolving at a certain temperature, and a modified solution is obtained after sol is filtered out. The concrete release agent has the beneficial effects that the concrete release agent is a water-based release agent, is nontoxic and free of pollution, can be sprayed and smeared, and is convenient to use; and the release agent can be degraded automatically within a certain period after smearing, a formwork does not need to be cleaned, time and labor are saved, and the cost performance is high.
Owner:大连市铭源全科技开发有限公司
Synthesis method and application of a multi-target Aβ oligomerization inhibitor
ActiveCN106831713BHigh yieldSuitable for mass manufacturingNervous disorderOrganic chemistrySynthesis methodsDibromopropane
Owner:SHENZHEN UNIV
Preparation method of (-)-Cytoxazone and (+)-4-epi-Cytoxazone
InactiveCN113636983AEasy to operateHigh stereoselectivityOrganic chemistry methodsLithium chlorideCytoxazone
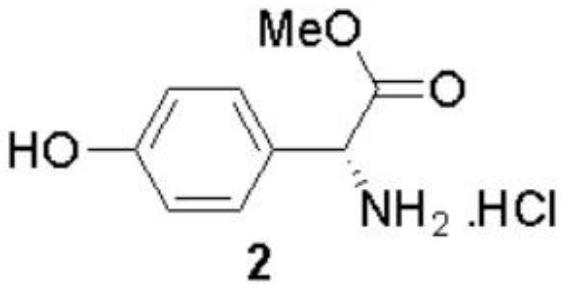
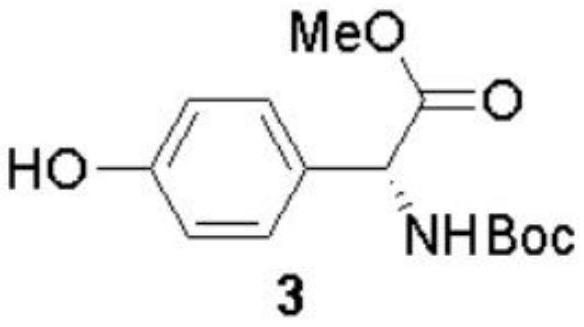
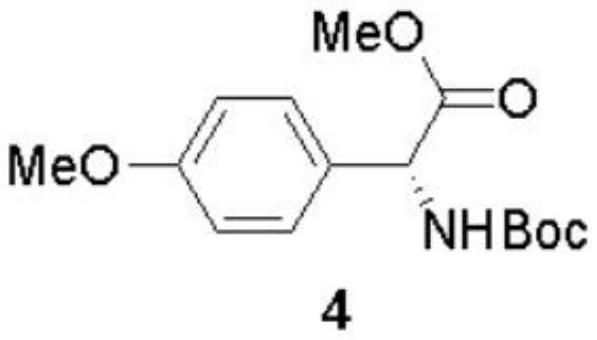
The invention discloses a preparation method of (-)-Cytoxazone and (+)-4-epi-Cytoxazone, which comprises the following steps: taking D-p-hydroxyphenylglycine as a raw material, carrying out methyl esterification reaction under the catalysis of thionyl chloride to obtain an intermediate 2, and then protecting amino with Boc anhydride to obtain an intermediate 3; taking potassium carbonate as alkali, and conducting reacting with methyl iodide under the reflux condition of acetonitrile to obtain a compound 4; reducing methyl ester by using a sodium borohydride / lithium chloride system to obtain a primary alcohol compound 5; and oxidizing primary alcohol by using IBX to obtain an intermediate 6, carrying out an SN2 reaction on the intermediate 6 and acetone cyanohydrin to obtain an intermediate 7, carrying out reflux by using a methanol solution of hydrogen chloride to obtain an intermediate 8, carrying out a reaction on the intermediate 8 and triphosgene to obtain two five-membered ring compounds 9 and 10, and carrying out reduction by using sodium borohydride to obtain (-)-Cytoxazone and an isomer (+)-4-epi-Cytoxazone. The synthetic route is convenient to operate.
Owner:SHENZHEN ELDERLY MEDICAL RES INST +1
Synthesis method of dimethyl octenone
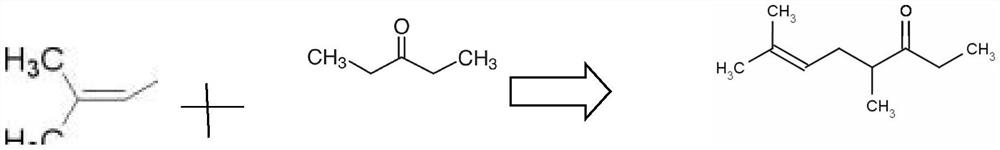
PendingCN112321403ASynthetic Method AdvantagesSimple process routeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHalohydrocarbonPtru catalyst
The invention provides a synthesis method of dimethyl octenone. The method comprises the following steps: (1) adding 3-pentanone, 50 wt% of basic catalyst, and a phase transfer catalyst into a four-neck flask with a stirring function, and heating to 20-50 DEG C while stirring; (2) dropwise adding 3, 3-dimethyl allyl bromide, continuously stirring for 1 hour after dropwise adding, sampling and detecting, stopping stirring when the 3, 3-dimethyl allyl bromide is completely reacted, and standing for layering to obtain an organic phase and a water phase; and (3) extracting the water phase obtainedin the step (2) by using 3-pentanone to obtain an organic phase, combining the organic phase with the organic phase obtained in the step (2), and distilling to obtain a dimethyl octenone finished product. According to the synthesis method of the dimethyl octenone, a bimolecular nucleophilic substitution reaction (SN2) of halogenated hydrocarbon is carried out, and a-site hydrogen of carbonyl is substituted with long-chain branched chain brominated hydrocarbon; the method is simple in process route, capable of being completed in one step and mild in condition.
Owner:TIANJIN ANKAITE CATALYST
Preparation method of food grade high viscosity sodium carboxymethyl starch
ActiveCN102229675AFacilitated DiffusionImprove permeabilityFood preservationFood preparationSolubilityReaction rate
The invention provides a preparation method of food grade high viscosity sodium carboxymethyl starch. Starch and chloroacetic acid undergo a bimolecular nucleophilic substitution reaction under alkaline conditions to produce a food grade high viscosity sodium carboxymethyl starch product. In the invention, through a solvent method, an alkalization process and an etherification process on starch can be carried out in slurry in the presence of an organic solvent as a reaction medium and through a refrigeration process on starch at low temperature, the infiltration and the penetration of starch in material liquid are promoted and a reaction speed, a main reaction rate, product properties of uniformity, transparency and solubility, and a product grade are improved.
Owner:HUBEI DAYA BIOLOGICAL TECH CO LTD
Precursor of key intermediate of AG 7088 class compound and its sythetic process
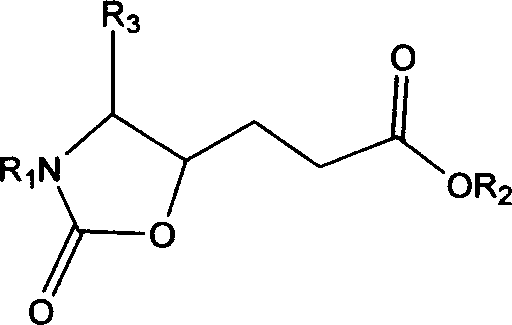
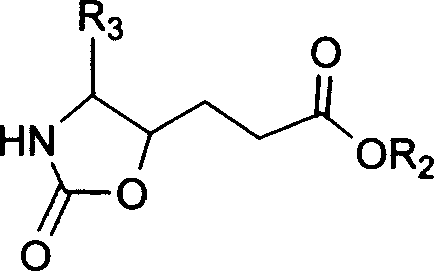
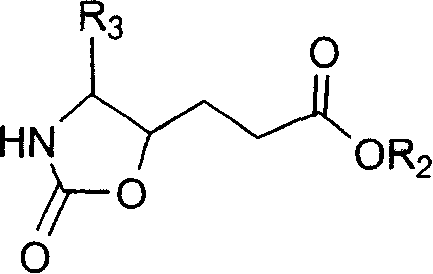
This invention relates to synthesis of precursor of intermediate of AG7088 type compounds, with its constructural formula as shown. Under N protection, coupling reaction of amino-aldehyde and propiolate is proceeded. Said product is hydrogenized with N protection base being substituted by Boc or other nucleophilic protection base. Said product is then hydroxylated activated. Then it is proceeded inner molecular SN2 reaction to produce oxazolon ane, then being reverse turning its hydroxy structure, adding N protection base, opening the ring of oxazolidone, under acid condition to close penta internal ester ring to obtain intermediate of AG7088. In this invention natural amino acid is used as raw material, with short synthesis process, high yield, good stereoselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
A kind of production method of modified cassava starch
Owner:GUANGXI JINHAN ENVIRONMENTAL PROTECTION CO LTD
Preparation method of food grade high viscosity sodium carboxymethyl starch
ActiveCN102229675BFacilitated DiffusionImprove permeabilityFood preservationFood preparationSolubilityReaction rate
The invention provides a preparation method of food grade high viscosity sodium carboxymethyl starch. Starch and chloroacetic acid undergo a bimolecular nucleophilic substitution reaction under alkaline conditions to produce a food grade high viscosity sodium carboxymethyl starch product. In the invention, through a solvent method, an alkalization process and an etherification process on starch can be carried out in slurry in the presence of an organic solvent as a reaction medium and through a refrigeration process on starch at low temperature, the infiltration and the penetration of starch in material liquid are promoted and a reaction speed, a main reaction rate, product properties of uniformity, transparency and solubility, and a product grade are improved.
Owner:HUBEI DAYA BIOLOGICAL TECH CO LTD
A kind of method for continuously preparing 4-(6-hydroxyhexyloxy)phenol
ActiveCN107867979BHigh yieldContinuous operationEther separation/purificationChemical/physical/physico-chemical microreactorsPtru catalystSN2 reaction
The invention discloses a method for continuously preparing 4-(6-hydroxyhexyloxy)phenol in a micro-reactor. The method comprises the following steps: dissoling hydroquinone and 6-chloro-1-hexanol in the solvent ethanol; and with an alkali aqueous solution of NaOH<-> as a catalyst, carrying out a bimolecular nucleophilic substitution reaction in the micro-reactor so as to continuously prepare 4-(6-hydroxyhexyloxy)phenol. Compared with the prior art, the method provided by the invention has the following advantages that the synthesis time of 4-(6-hydroxyhexyloxy)phenol is reduced to 10 min or less from original 20 hours; yield is increased from original 40% to 80%;continuous operation during the reaction can be realized; and integration and enlargement can be easily realized.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A kind of preparation method of green chelating agent
ActiveCN109503402BReduce usageImprove product qualityOrganic compound preparationAmino-carboxyl compound preparationChemical industryPtru catalyst
The invention provides a preparation method of a green chelating agent. Specifically, under normal pressure conditions, the use of partially neutralized amino acids and chloroacetic acid produces S under the action of a catalyst. N 2 Bimolecular nucleophilic substitution reaction to prepare a series of green chelating agents, wherein the catalyst is iodide of alkali metal, and the amount of catalyst used is 0.01-0.30% of the input mass of amino acid. The method of the present invention adopts an atmospheric pressure catalytic reaction system, the catalyst usage is small, and the treatment method is simple; the catalytic performance is good, and the product yield can reach more than 96%; Chemical industry production.
Owner:SHANDONG TAIHE WATER TREATMENT TECH CO LTD
Maleate monomers used for preparing pour depressant components
InactiveCN107759470AEasy to prepareRaw materials are easy to getOrganic compound preparationCarboxylic acid esters preparationAlkaneAlcohol
The invention discloses maleate monomers used for preparing pour depressant components and a preparation method of the maleate monomers. The maleate monomers are structurally characterized by being maleate alkoxy alcohol ester. The preparation method comprises the steps that n-alcohol and n-alkane with the terminal substituted with dichloro are used as raw materials, chloroether with the total number of carbon of 20-26 is synthesized through the Williamson ether synthesis method, and then the chloroether and sodium maleate are subjected to the SN2 reaction to synthesize maleate. The preparation method of the maleate monomers is simple, the raw materials are easy to obtain, the product yield is high, and many kinds of maleate monomers can be prepared.
Owner:天津市明瑞石油科技有限公司
A kind of preparation method of tetrasodium aspartic acid diacetate
ActiveCN109400492BReduce usageImprove product qualityOrganic compound preparationAmino-carboxyl compound preparationReaction rateSN2 reaction
The invention provides a novel preparation method of tetrasodium aspartic acid diacetate. Specifically, under certain temperature conditions, the partially neutralized natural amino acid L-aspartic acid is used as a raw material, and SN2 bimolecular nucleophilic substitution reaction occurs with chloroacetic acid under alkaline conditions to prepare tetrasodium aspartic acid diacetate. Among them, chloroacetic acid and sodium hydroxide are added dropwise into the kettle, and the pH value of the reaction system is controlled in the range of 9~12. At the same time, the reaction process is under the condition of 0~-0.005MPa negative pressure, and the water in the reaction system is removed in time to maintain Reaction material concentration, improve conversion rate and reaction rate. The method of the invention has short reaction period, simple production process, and the yield can reach 99%. The produced water can be recycled, no toxic by-products and waste water are produced, safe and environment-friendly, and easy to realize large-scale industrial production.
Owner:SHANDONG TAIHE WATER TREATMENT TECH CO LTD
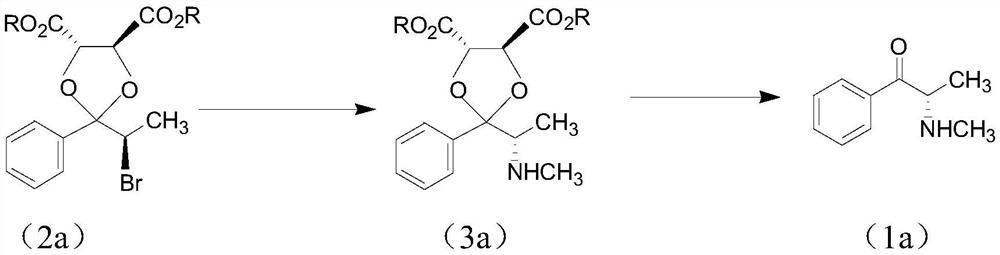
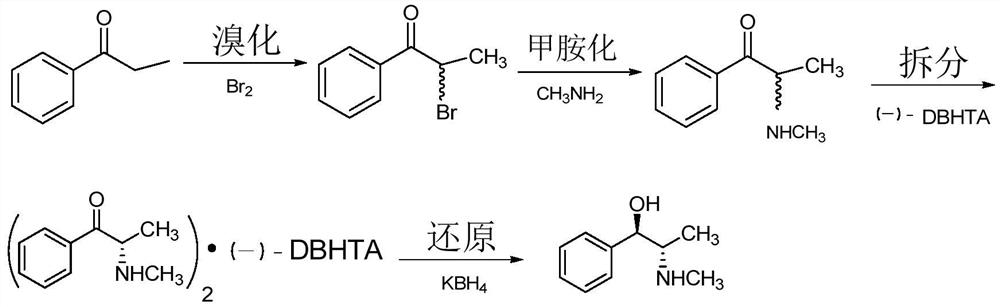
Chiral synthesis method of ephedrine key intermediate (S)-2-methylamino-1-phenyl-1-acetone
ActiveCN112645829AHigh yieldHigh purityOrganic compound preparationOrganic chemistry methodsPotassium borohydrideEphedrine
The invention discloses a chiral synthesis method of an ephedrine key intermediate (S)-2-methylamino-1-phenyl-1-acetone. The method comprises the following steps: carrying out nucleophilic substitution reaction on dimethyl-(4S, 5S)-2-[(R)-1-bromoethyl]-2-phenyl-1, 3-dioxyethane-4, 5-dicarboxylate (2a) and methylamine in an SN2 reaction environment to finish Walden inversion to generate dimethyl-(4S, 5S)-2-[(S)-1-methylamino]-2-phenyl-1, 3-dioxyethane-4, 5-dicarboxylate(3a); and then removing a chiral auxiliary agent (2S, 3S)-dimethyl tartrate to obtain (S)-2-methylamino-1-phenyl-1-acetone (1a). The compound can be used as a substrate for potassium borohydride reduction in the ephedrine preparation process, so that traditional complicated physical and chemical coexisting splitting operation of dibenzoyl tartaric acid is avoided, and the method has an important industrial application value.
Owner:ZHEJIANG APELOA KANGYU PHARMA +1
A kind of sulfonate type fluorosilicon anionic surfactant and preparation method thereof
ActiveCN109173922BGood water solubilityPromote degradationSilicon organic compoundsTransportation and packagingPolymer scienceActive agent
The invention discloses a sulfonate-type fluorosilicon anionic surfactant and a preparation method thereof. The preparation method uses an easily degradable, low-toxic fluorine-containing compound C 6 f 13 SO 2 F as the starting point, firstly, the bimolecular nucleophilic substitution (SN2) reaction with N-methallylamine was carried out to prepare NSF6, and then NSF6 was sequentially reacted with hydrogen-containing silicone oil (PMHS) and allyl glycidyl ether (AGE) for hydrosilation reaction, Finally with NaHSO 3 Sulfonate-type fluorosilicon anionic surfactant PSF6AS was synthesized by sulfonation reaction, and its surface tension and critical micelle concentration were measured. Compared with traditional fluorocarbon surfactants, this surfactant has better water solubility and biodegradability; it also provides a new research method and idea for the development and application of sulfonate-type fluorosilicon anionic surfactants in my country .
Owner:JIUJIANG UNIV
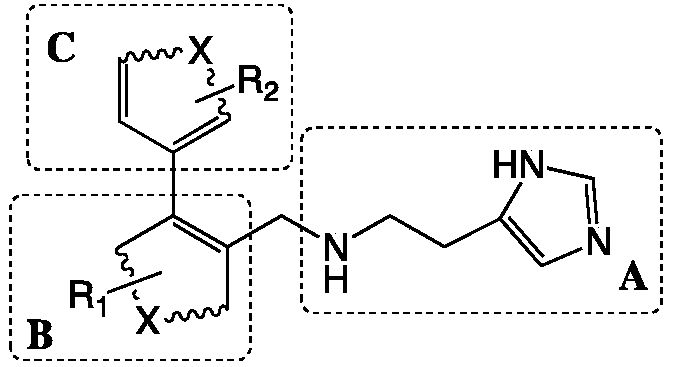
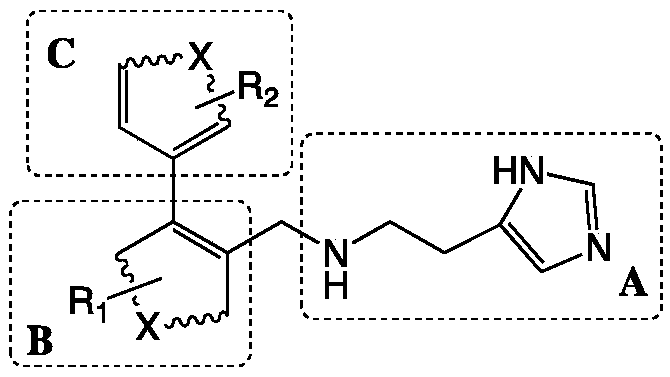
Preparation method and application of 4-imidazolyl-containing glutaminyl cyclase inhibitor
ActiveCN109305942AHigh activitySimple process routeNervous disorderOrganic chemistryDiseasePharmacophore
The invention discloses a preparation method and application of a 4-imidazolyl-containing glutaminyl cyclase inhibitor. The preparation method comprises the following steps that a B unit raw materialwith a bromine substituent group and R1 and a C unit raw material with a boric acid group and R2 are firstly subjected to Suzuki coupling reaction so as to prepare a B unit and C unit coupled biphenylintermediate; and the B unit and C unit coupled biphenyl intermediate and a 2-(1H-imidazole-4-yl)ethylamine serve as raw materials and are subjected to SN2 reaction so as to prepare a compound basedon 4-imidazolyl pharmacophore. According to the preparation method, preparation can be completed only through the two steps of Suzuki coupling reaction and SN2 reaction, the process route is simple and feasible, the yield is high, and the method is more suitable for large-scale preparation; and the 4-imidazolyl-containing glutaminyl cyclase inhibitor prepared through the preparation method has higher activity and can be widely applied to development of new drugs such as high-efficiency QC inhibitor, development of drugs for AD, tumor, rheumatoid arthritis and other QC specificity high-expression related diseases and early diagnosis and diagnosis kits, and the like.
Owner:SHENZHEN UNIV
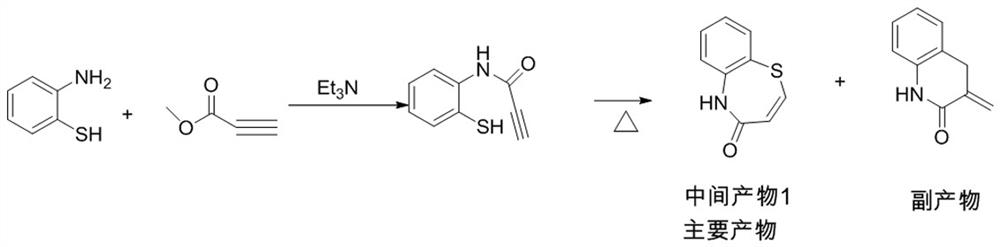
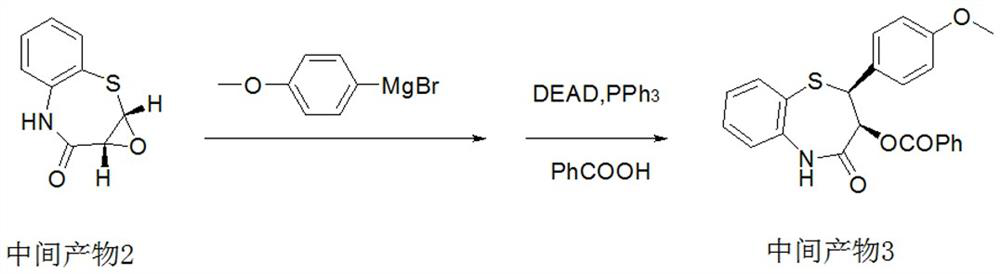
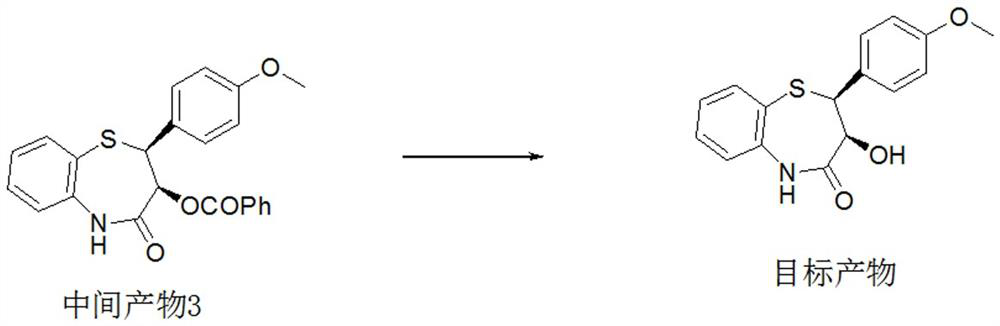
Preparation method of 2-(4-methoxyphenyl)-3-hydroxy-2, 3-dihydro-1, 5-benzothiazepine ketone
The invention provides a preparation method of 2-(4-methoxyphenyl)-3-hydroxy-2, 3-dihydro-1, 5-benzothiazepine ketone. The preparation method comprises the following steps: taking o-aminothiophenol and methyl propiolate as raw materials to synthesize an intermediate product 1, carrying out selective epoxidation to obtain an intermediate product 2, reacting the intermediate product 2 with 4-methoxyphenyl anions, carrying out Mitsunobu reaction to obtain an intermediate product 3, and hydrolyzing to obtain the product. In the cyclization process of the intermediate product 1, the tension of Z-structure olefin is small so that the yield is high, subsequent epoxidation is an asymmetric epoxidation reaction, the obtained intermediate product 2 is high in optical purity and high in yield, the intermediate product 2 and 4-methoxyphenyl anions are subjected to SN2 reaction, configuration inversion is carried out in an ortho-position, and the product is obtained. The whole reaction process doesnot need to be split, the total yield of the route is high, and the method is economical.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Precursor of key intermediate of AG 7088 class compound and its sythetic process
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com