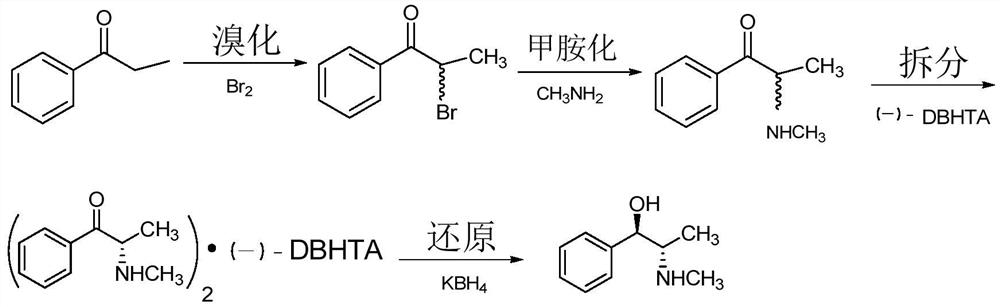
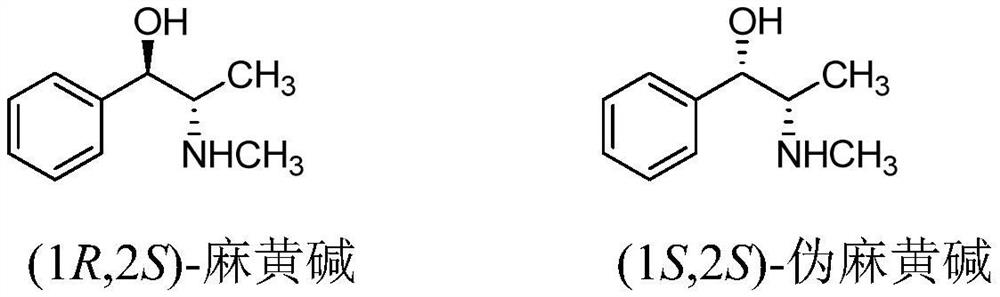
Chiral synthesis method of ephedrine key intermediate (S)-2-methylamino-1-phenyl-1-acetone
A technology of chiral synthesis and methylamine group, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of not obtaining products, complexity, etc., and achieve improved yield and purity, simplified operations, and Avoid the effect of splitting the course of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
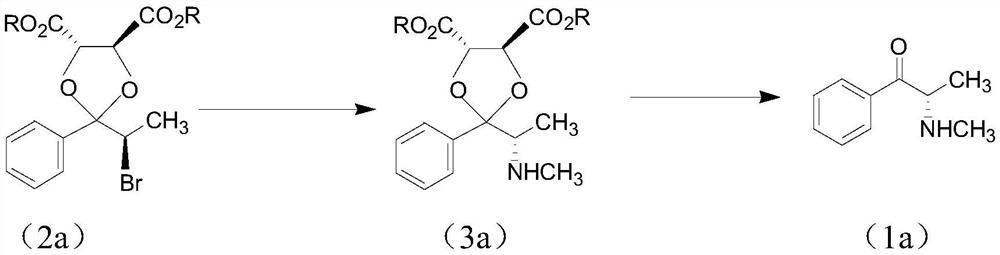
[0045] The first step reaction: chiral ketalization of the α-position carbonyl of propiophenone
[0046]
[0047] Propiophenone (125mmol), (2S,3S)-(-)-bismethyl tartrate diester (130mmol) and trimethyl orthoformate (250mmol) were mixed and heated to 50°C, then methanesulfonic acid (8mmol) was added . Incubate at 95°C for five days. The post-processing process was completed with reference to J.Chem, Soc.Perkin Trans, I.2759-2765 (2000). Obtain 35 g of oily product (4), with a yield of 95%.
Embodiment 2
[0049] The second step reaction: α-stereoselective bromination of ketal (4)
[0050]
[0051] Ketal (4) (170mmol) was dissolved in 260ml of 1,2-dichloroethane, then cooled to -10°C. Bromine (28.5g, 176mmol) was dissolved in 48mL of 1,2-dichloroethane, and slowly added dropwise to the 1,2-dichloroethane reaction liquid in (4) above, and the post-treatment was completed according to the method provided in the above-mentioned literature . 61.3 g of (4S,5S)-2-[(R)-1-bromoethyl]-2-phenyl-1,3-dioxyethane-4,5-dicarboxylate (2a) was obtained. The ratio is 97%, and the de value of R / S is 93%.
Embodiment 3
[0053] The third step reaction: Walden SN of bromide (2a) 2 Inversion, chiral preparation of methylamine (3a) (absorption of HBr with excess methylamine)
[0054]
[0055] Bromide (2a) (44.8g, 120mmol) was dissolved in anhydrous acetonitrile (447ml), cooled to -15°C with dry ice acetone solution, and the anhydrous dried methylamine gas was slowly passed into the reaction vessel until the solution increased in weight It is 7.5g, that is, the weight of methylamine is 242mmol. Close the reactor, then slowly raise the temperature to 20-30°C, and keep it warm for 14 hours. After the reaction was completed, the reactor was opened to release the pressure. Acetonitrile was distilled off under reduced pressure. 300 ml of dichloromethane was added to the residue, and the layers were washed with water three times (80 ml of water each time) to remove the methylammonium salt of hydrogen bromide. The separated dichloromethane solution was dried over anhydrous magnesium sulfate, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com