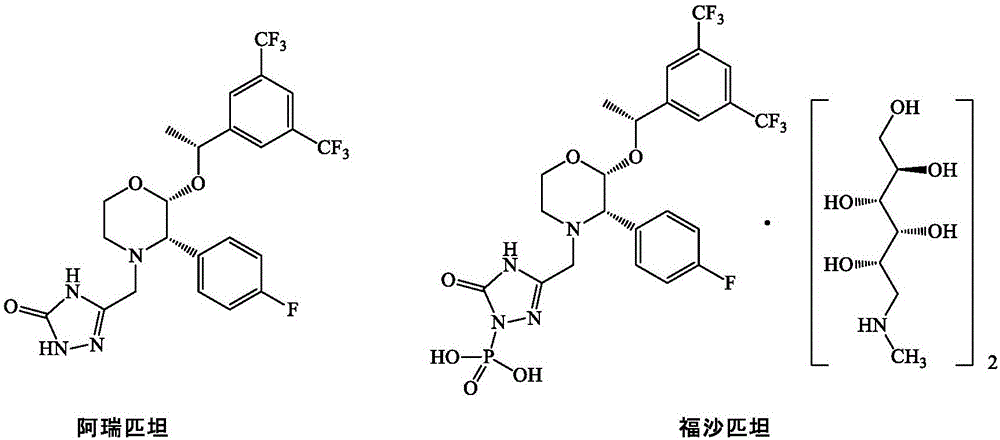
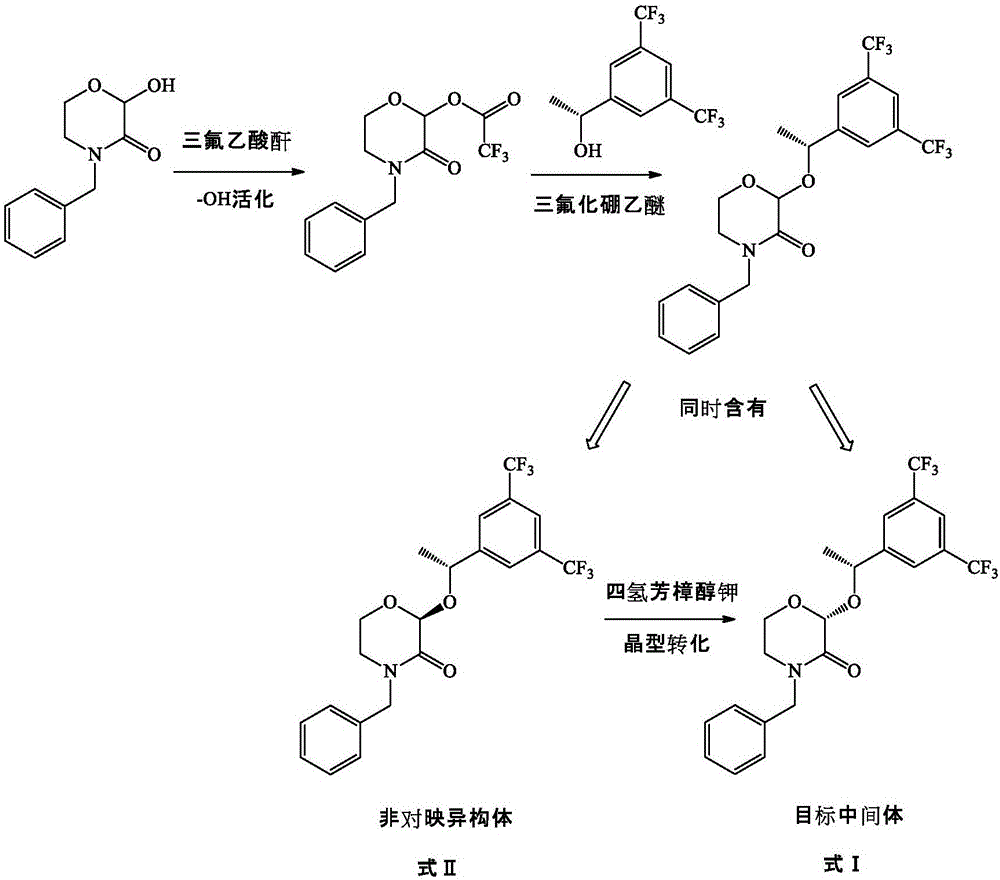
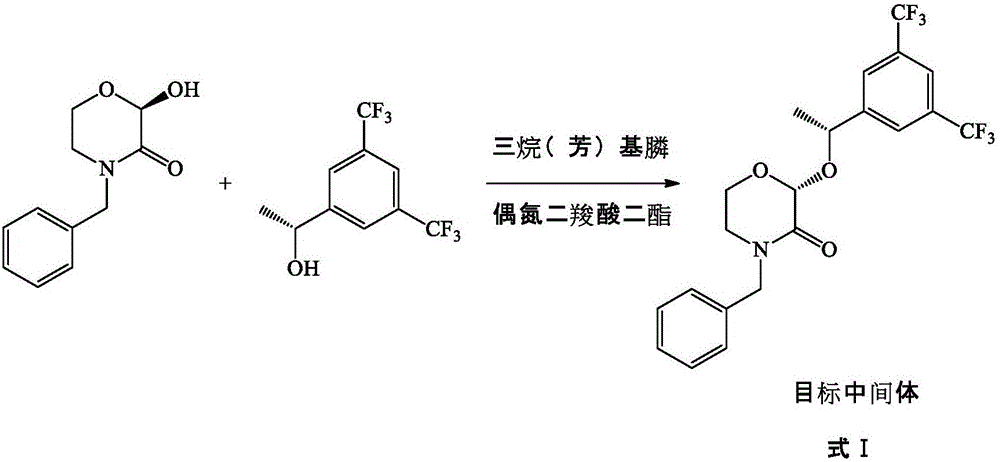
Preparation method of morpholine derivative
A morpholine derivative and morpholine technology, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of increasing the difficulty of reaction treatment, not suitable for industrial production, long process, etc., to achieve mild conditions, reduce difficulty, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (R)-4-benzyl-2-hydroxy-morpholin-3-one (3.11g, 15mmol), (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethyl -1-ol (4.65 g, 18 mmol) and triphenylphosphine (4.72 g, 18 mmol) were dissolved in 150 mL of tetrahydrofuran. Below 10°C, a solution of diethyl azodicarboxylate in tetrahydrofuran (3.13 g, 18 mmol of diethyl azodicarboxylate dissolved in 20 mL of tetrahydrofuran) was slowly added dropwise. After the dropwise addition, the reaction solution was raised to room temperature and continued to stir for 12 h. Concentrate the reaction solution to dryness under reduced pressure, add 100 mL of n-hexane and 100 mL of water, heat to 50°C, and stir well. Stand still, separate the liquids, dry the upper organic phase with anhydrous sodium sulfate, distill off a large amount of n-hexane under reduced pressure, cool the remaining solution to 0°C for crystallization, filter with suction, wash the obtained solid with ice n-hexane (25mL×2), After air drying at 40°C, 5.84 g of a white solid...
Embodiment 2
[0045] (R)-4-benzyl-2-hydroxy-morpholin-3-one (3.11g, 15mmol), (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethyl -1-ol (15.49 g, 60 mmol) and triphenylphosphine (15.74 g, 60 mmol) were dissolved in 150 mL of tetrahydrofuran. Below 10°C, a solution of diethyl azodicarboxylate in tetrahydrofuran (10.45 g, 60 mmol of diethyl azodicarboxylate dissolved in 20 mL of tetrahydrofuran) was slowly added dropwise. After the dropwise addition, the reaction solution was raised to room temperature and continued to stir for 12 h. Concentrate the reaction solution to dryness under reduced pressure, add 100 mL of n-hexane and 100 mL of water, heat to 50°C, and stir well. Stand still, separate the liquids, dry the upper organic phase with anhydrous sodium sulfate, distill off a large amount of n-hexane under reduced pressure, cool the remaining solution to 0°C for crystallization, filter with suction, wash the obtained solid with ice n-hexane (25mL×2), Air-dried at 40°C to finally obtain 5.95 g of ...
Embodiment 3
[0050] (R)-4-benzyl-2-hydroxy-morpholin-3-one (3.11g, 15mmol), (R)-1-(3,5-bis(trifluoromethyl)phenyl)ethyl -1-ol (7.75 g, 30 mmol) and triphenylphosphine (7.87 g, 30 mmol) were dissolved in 150 mL of dichloromethane. Below 10°C, a solution of di-p-chlorobenzyl azodicarboxylate in dichloromethane (11.02 g, 30 mmol of di-p-chlorobenzyl azodicarboxylate dissolved in 20 mL of dichloromethane) was slowly added dropwise. After the dropwise addition, the reaction solution was raised to room temperature and continued to stir for 12 h. Concentrate the reaction solution to dryness under reduced pressure, add 100 mL of n-hexane and 100 mL of water, heat to 50°C, and stir well. Stand still, separate the liquids, dry the upper organic phase with anhydrous sodium sulfate, distill off a large amount of n-hexane under reduced pressure, cool the remaining solution to 0°C for crystallization, filter with suction, wash the obtained solid with ice n-hexane (25mL×2), Air-dried at 40°C to finally...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com