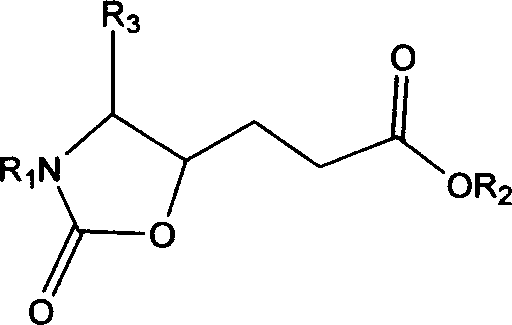
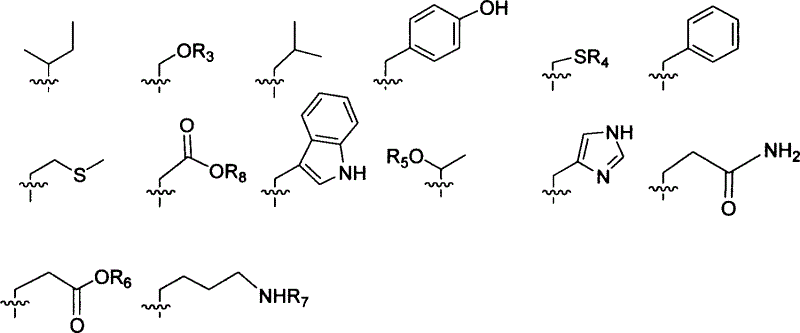
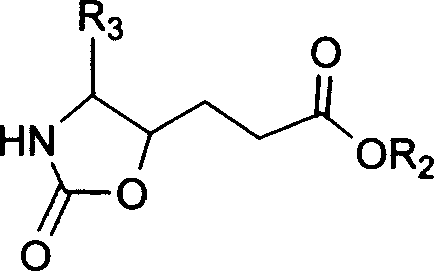
Precursor of key intermediate of AG 7088 class compound and its sythetic process
A technique for the synthesis of AG7088, which is applied in the direction of organic chemistry, can solve the problems of low selectivity and low yield, and achieve the effects of high yield, easy availability of raw materials, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Coupling
[0050] Under the protection of argon at -78°C, 2.5ml (4mmol) of BuLi was slowly added dropwise to 0.67ml (4.8mmol) of diisopropylamine dissolved in 6ml of THF, and after stirring for half an hour, 0.46ml was slowly added dropwise. (4mmol) of ethyl propiolate, reacted for half an hour, slowly added 593mg (2.1mmol) of N-dibenzyl galenaldehyde dissolved in 4ml TFH, and added saturated NH 4 Aqueous Cl solution quenched the reaction. The temperature of the reaction system was allowed to slowly rise to room temperature. Then add 10ml of 1M aqueous citric acid solution, then extract the product with 15ml of ether, and then use saturated NaHCO 3 Wash the organic phase with NaCl aqueous solution, separate the organic layer, anhydrous NaCl 2 SO 4 Drying, concentration, and column chromatography yielded 489 mg of a yellow viscous liquid with a yield of 73%. At the same time, 94 mg of another isomer was obtained, and the ratio betw...
Embodiment 2
[0055] Example 2: Hydrogenation
[0056] 437 mg of the product obtained in the first step was dissolved in 5 ml EtOAc, and 512 mg of (Boc) 2 O 175 mg of Pd(OH) 2 , hydrogenated at 40° C. under 50 atm pressure for one day, evaporated the solvent under reduced pressure, and obtained 315 mg of the product by flash column chromatography, with a yield of 90%.
[0057]
[0058] The spectrogram data is as follows:
[0059] 1 H NMR: δ0.93(d, 3H, J=6.6), 1.26(d, 3H, J=6.6), 1.26(t, 3H, J=7.2), 1.45(s, 9H), 1.56-1.67(m , 1H), 1.77-1.93(m, 2H), 2.50-2.56(ddd, J 1 =2,7,J 2 =6.9, J3=9.0), 2.92 (d, 1H, J=6.3) 3.53-3.46 (m, 1H), 3.61-3.71 (m, 1H), 4.14 (q, J=7.2), 4.45, (d, J=9.3).
[0060] ESI-Ms: [M+1]=304.2
[0061] Optical rotation: CHCl 3 , c=1.070, [α]=-9.3
Embodiment 3
[0062] Example 3: Hydroxyl Flip Ring Closure
[0063] Dissolve 91 mg of the product from the previous step in 2.5 ml of CH 2 Cl 2 208 μl of NEt were sequentially cooled in an ice-salt bath under the protection of argon 3 , 58μl of MsCl was added to it, reacted for half an hour, and added 5ml of CH 2 Cl 2 Dilute sequentially with 1M HCl, saturated NaHCO 3 and NaCl aqueous solution, the organic layer was separated, anhydrous NaSO 4 Dry and concentrate. The obtained product was dissolved in 4 ml of DMF and heated to 65° C. for one day without isolation. DMF was distilled off under reduced pressure and flash column chromatography was used to obtain 59 mg of the product, with a two-step yield of 86%.
[0064]
[0065] The spectrogram data is as follows:
[0066] 1 H NMR δ 0.93 (d, 3H, J=5.4), 0.95 (d, 3H, J=5.4), 1.27 (t, 3H, J=7.2), 1.67-1.79 (m, 1H), 1.92-2.04 ( m, 2H), 2.53(t, 2H, J=4.2), 3.19(t, 1H, J=5.1), 4.15(q, 2H, J=7.2), 4.32-4.36(m, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com