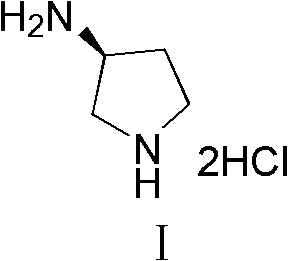
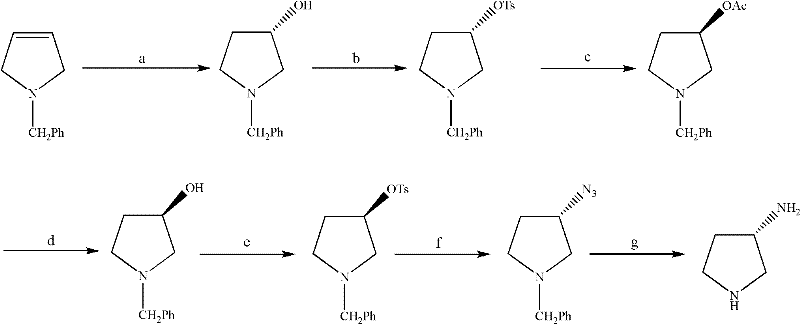
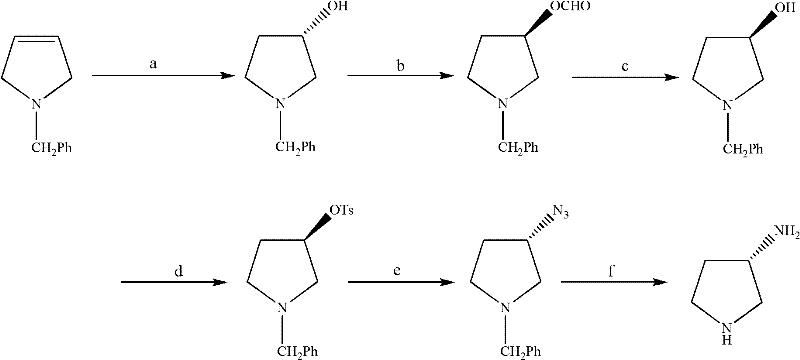
Synthesis method of (S)-3-amino pyrrolidine dihydrochloride
A technology of aminopyrrolidine dihydrochloride and hydroxypyrrolidine hydrochloride, which is applied in the field of preparation of chiral drugs, can solve the problems of difficult recovery of solvent THF and cumbersome operation, and achieve low cost, readily available raw materials, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (R)-3-hydroxypyrrolidine hydrochloride (II) preparation
[0042] Mix trans-4-hydroxyl-L-proline (100.0g, 0.75mol), cyclohexanol (500.0ml) and 2-cyclohexen-1-one (10.0ml, 0.11mol) and heat to 154°C, heat preservation reaction for 5h, after cooling to room temperature, add toluene (500ml), cool in an ice-water bath to about 0°C, pass in dry hydrogen chloride gas until the pH value is 2-3, stir at about 5°C for 1h, and filter to obtain a solid , recrystallized from isopropanol (300ml) to give light pink crystalline solid II (75.0g, 80.0%), mp: 104~107°C
[0043] Yield: 80.0%
[0044] Preparation of (R)-1-tert-butoxycarbonyl-3-methanesulfonyloxypyrrolidine (III)
[0045] Light pink crystalline solid II (12.4g, 0.10mol) was placed in dichloromethane (200ml) solution, cooled to 0°C, triethylamine (18.0ml, 0.13mol) was added, after stirring evenly, dropwise added ( Boc) 2 O (22.0g, 0.10mol) in dichloromethane (100ml) solution, reacted at 0°C for 3h, then added triethylamin...
Embodiment 2
[0058] (R)-3-hydroxypyrrolidine hydrochloride (II) preparation
[0059] Mix trans-4-hydroxyl-L-proline (10.0g, 0.75mol), cyclohexanol (50.0ml) and 2-cyclohexen-1-one (1.0ml, 0.11mol) and heat to 150°C, heat preservation reaction for 3h, after cooling to room temperature, add toluene (50ml), cool in an ice-water bath to about 0°C, pass in dry hydrogen chloride gas until the pH value is 2-3, stir at about 5°C for 1h, and filter to obtain a solid , recrystallized from isopropanol (30ml) to give light pink crystalline solid II (7.7g, 82.1%), mp: 104~107°C
[0060] Yield: 82.1%
[0061] Preparation of (R)-1-tert-butoxycarbonyl-3-methanesulfonyloxypyrrolidine (III)
[0062] Put light pink crystalline solid II (62.1g, 0.50mol) in dichloromethane (800ml) solution, cool to 0°C, add triethylamine (90.0ml, 0.65mol), stir evenly, add dropwise ( Boc) 2 O (110.0g, 0.50mol) in dichloromethane (400ml) solution, reacted at 0°C for 3h, then added triethylamine (80.0ml, 0.55mol), then added ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com