Preparation method of (-)-Cytoxazone and (+)-4-epi-Cytoxazone
A technology of inhibitors and cells, applied in the field of medicine, can solve problems such as poor stereoselectivity, harsh reaction conditions, and low total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
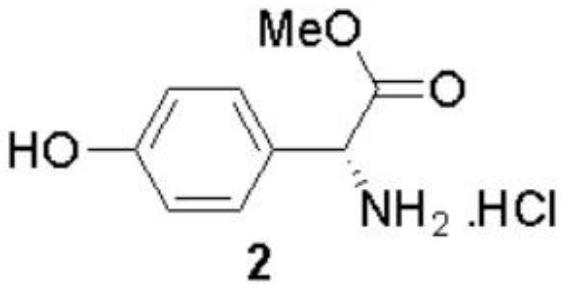
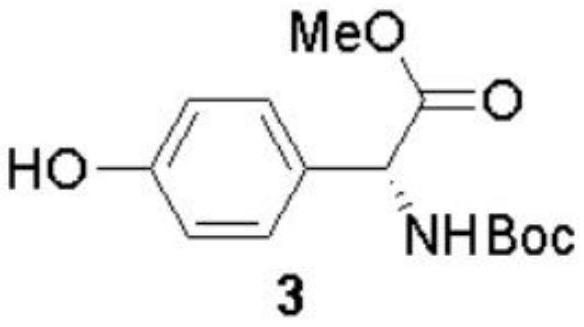
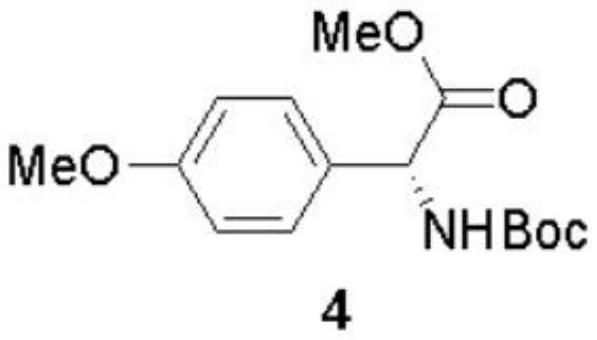
[0090] The preparation method of (-)-Cytoxazone and (+)-4-epi-Cytoxazone of the present invention uses D-p-hydroxyphenylglycine as the starting material, and finally obtains the target product through four major steps. The reaction scheme is as follows:
[0091]
[0092] (1) Synthesis of compound 4
[0093] Dissolve compound 1 (D-p-hydroxyphenylglycine, 10g, 60mmol) in methanol (150mL), cool in an ice-water bath to 0°C, slowly add thionyl chloride (13mL, 180mmol) dropwise, and heat up to reflux after 30 minutes of dropping After reacting for 3h, it was concentrated under reduced pressure to obtain intermediate 2.
[0094]
[0095] Intermediate 2 was dissolved in tetrahydrofuran / water mixed solvent (1:1, 200mL), solid sodium bicarbonate (15.1g, 180mmol) and equivalent Boc anhydride (13.8mL, 60mmol) were added, reacted at room temperature for 10h, and Concentrate under reduced pressure, extract three times with ethyl acetate (200 mL), combine the organic phases, wash wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com