Preparation method and intermediate of lepimectin
A time and compound technology, applied in the field of pesticides, can solve the problems of low milbemycin fermentation unit, unsuitable for industrialized large-scale production, and low total process yield, and achieve the effects of simplifying operations, reducing production costs, and shortening reaction routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The synthesis of embodiment 1 2-methoxyiminophenylacetic acid
[0075] Dissolve 50g (0.030mol) of ethyl benzoylformate in 350mL of methanol solution containing 33g of methoxyamino hydrochloride, reflux at 80°C for 8h, TLC detects that the reaction is complete, extract with ethyl acetate (3×100mL), and concentrate Separation by silica gel column chromatography (elution system: methanol: chloroform = 1:9 (v / v) to obtain 53.6 g of ethyl 2-methoxyiminophenylacetate with a yield of 91%.
[0076] Dissolve 53.6g of ethyl 2-methoxyiminophenylacetate in 500mL of tetrahydrofuran and water mixture (v:v=1:1), slowly add 15mL of lithium hydroxide monohydrate, stir at 25°C for 5h, add 10M Hydrochloric acid was neutralized to neutral, extracted with ethyl acetate (3×300mL), the organic layer was washed with water and saturated brine, dried over anhydrous sodium sulfate, distilled to dryness under reduced pressure, and separated by silica gel column chromatography (elution system was e...
Embodiment 7
[0097] The preparation of embodiment 7 formula V-2 compound (lepinmycin A4)
[0098]
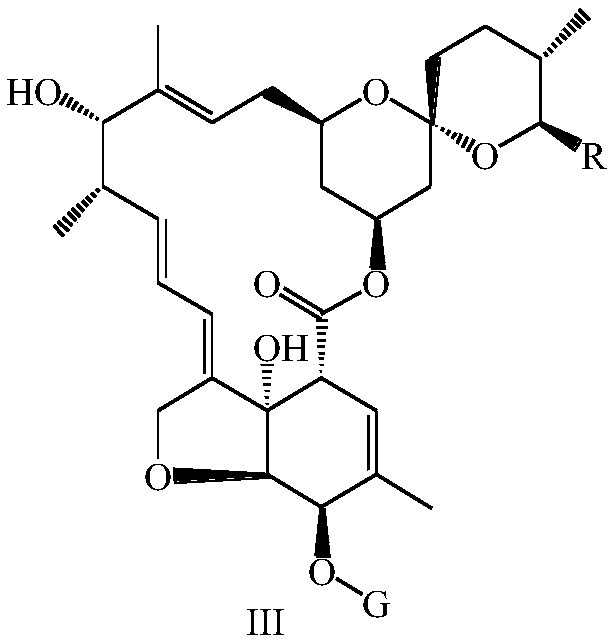
[0099] 3.2g (0.0167mol) of 2-methoxyiminophenylacetic acid, 5.90g (0.0087mol) of the compound of formula III-2 and 12g (0.0457mol) of triphenylphosphine were dissolved in 200mL toluene and tetrahydrofuran (5:1, v / v) into the mixed solution, add 10g (0.0574mol) diethyl azodicarboxylate dropwise at 0°C, after the dropwise addition, return to 10°C and stir for 3h, dilute with diethyl ether or n-hexane, filter to remove three Phenylphosphine oxide, the filtrate was concentrated under reduced pressure, the crude product was subjected to silica gel column chromatography, and the elution system was ethyl acetate:n-hexane=10:90 (v / v), to obtain 43.58 g of the compound of formula IV-2, ESI-MS : m / z 834.4[M+H] + , yield 49%.
[0100] 2g (0.0024mol) of the compound of formula IV-2 was added to 120mL of 1% (w / v) methanol solution of p-toluenesulfonic acid (1.2g, 0.007mol) in an ice-water bath, and...
Embodiment 8
[0105] The preparation of embodiment 8 Lepingmycin
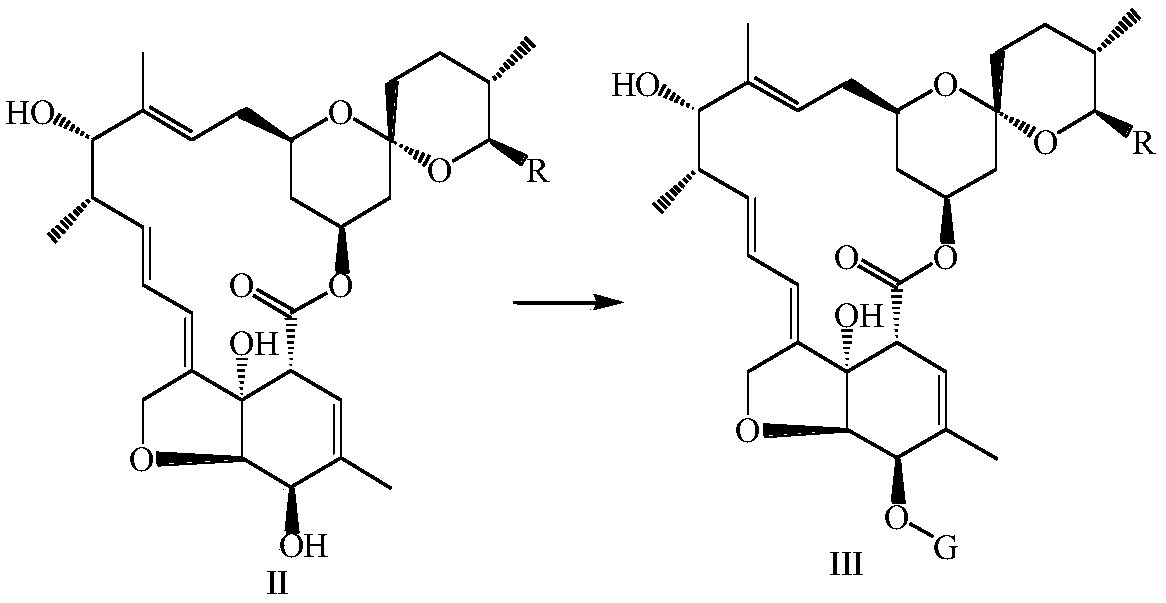
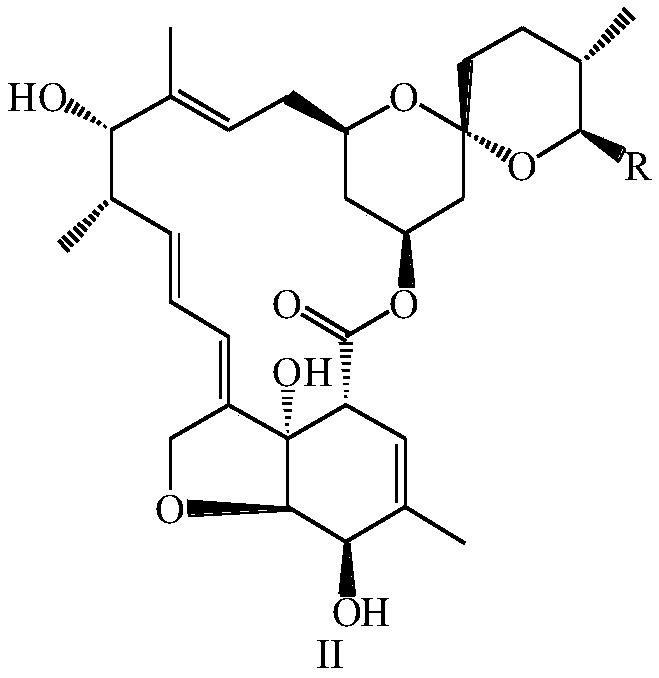
[0106] 5g of asvermectin was added to 75mL of methanol and 95% sulfuric acid (90:10, v / v) mixed solution, under nitrogen protection, stirred at 25°C for 10h, then added 40mL of ice water for dilution, dichloromethane extraction ( 3 × 80mL), the organic layer was washed with saturated sodium bicarbonate solution and clear water, the organic layers were combined, dried over anhydrous sodium sulfate, filtered, distilled under reduced pressure at 45°C, separated by silica gel column chromatography, and the elution system was ethyl acetate:petroleum Ether = 1:3 (v / v), to obtain 3.23 g of a mixture of the compound of formula II-1 and the compound of formula II-2, with a yield of 98%.
[0107] Dissolve 2g of the mixture of the compound of formula II-1 and the compound of formula II-2 in 25mL of dry dichloromethane, add 1.1g of imidazole, stir at 25°C until all the raw materials are dissolved, then add 1.65g (0.011mol) of tert-butyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com