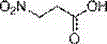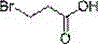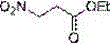Method for preparing 3-nitropropionic acid
A technology of nitropropionic acid and ethyl nitropropionate, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of high production cost and expensive reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: comprise the steps:
[0046] 1) Preparation of bromopropionic acid 4:
[0047] Add acrylic acid (162 g, 2.25 mol) and 47% HBr (400 g, 2.32 mol) into a 1L four-necked flask, and heat to reflux for 4 h. The water dispenser evaporates most of the water in the solution, and stops heating when the temperature reaches 130°C. Cool to form a white solid, add water to dissolve, extract with EA (150 ml×3), combine the organic phases to dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain 308 g of white solid with a yield of 90%.
[0048] 2) Preparation of ethyl 3-bromopropionate 3 :
[0049] 3-bromopropionic acid 4 (15 g, 100 mmol) was added to 200 ml of ethanol, under stirring, 2 droppers of concentrated sulfuric acid were added, and heated to reflux for 6 h. Ethanol was distilled off under reduced pressure, sulfuric acid was washed away by adding 150 ml of water, extracted with EA (200 ml×3), the organic phases were combined,...
Embodiment 2
[0054] Example 2: The difference from the examples is the difference in step 4): 3-ethyl nitropropionate2 (1 g, 6.8 mmol) to 10 ml THF+HO 2 O, LiOH was added in batches at room temperature for 5 h. Water was added and extracted with EA (100 ml×3), the organic phases were combined and dried over anhydrous sodium sulfate, distilled under reduced pressure, and 0.26 g of white solid was obtained by silica gel column chromatography, with a yield of 33%.
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com