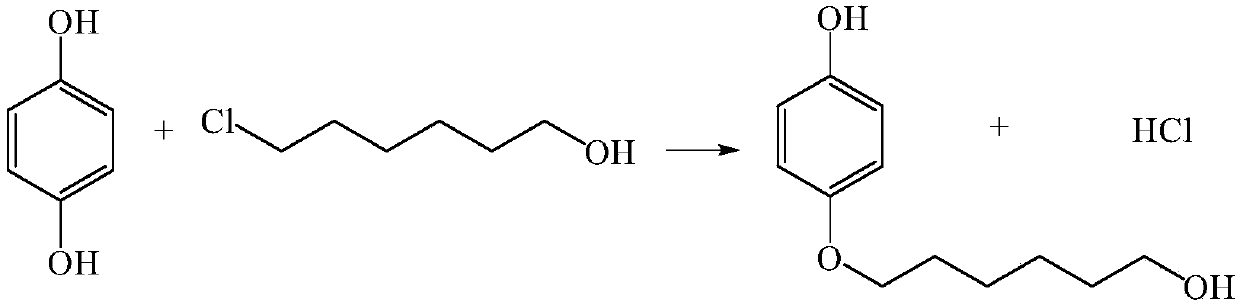
Method for continuously preparing 4-(6-hydroxyhexyloxy)phenol
A technology of hydroxyhexyloxyl and phenol, which is applied in the field of continuous preparation of 4-phenol using microreactor technology, can solve the problems of long production cycle, cumbersome operation process, and low yield, and achieve low process cost and easy integration and amplification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
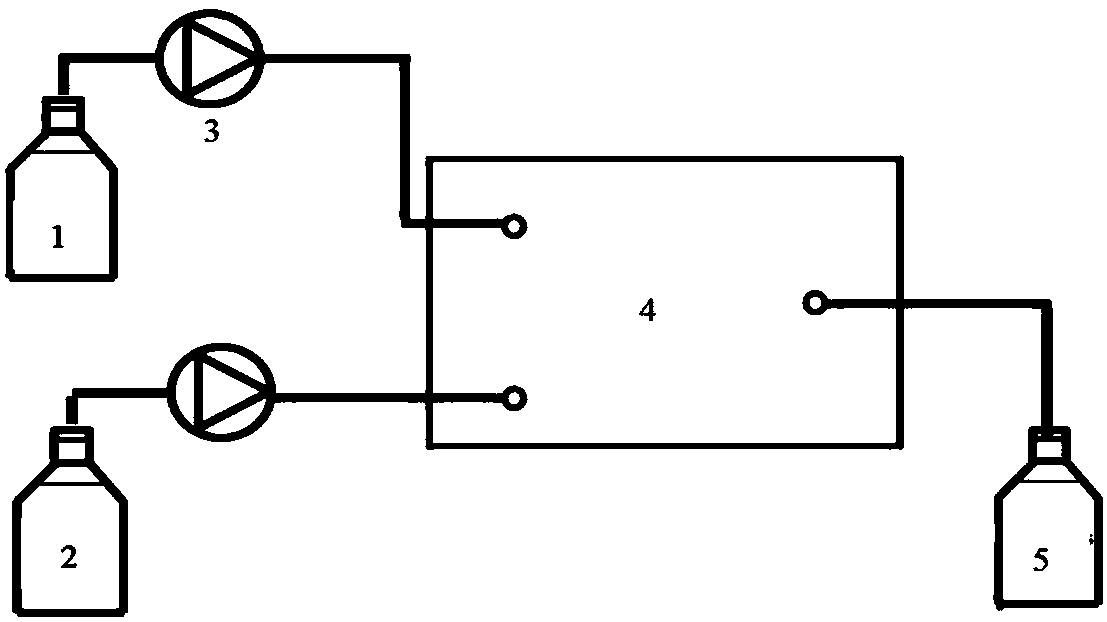
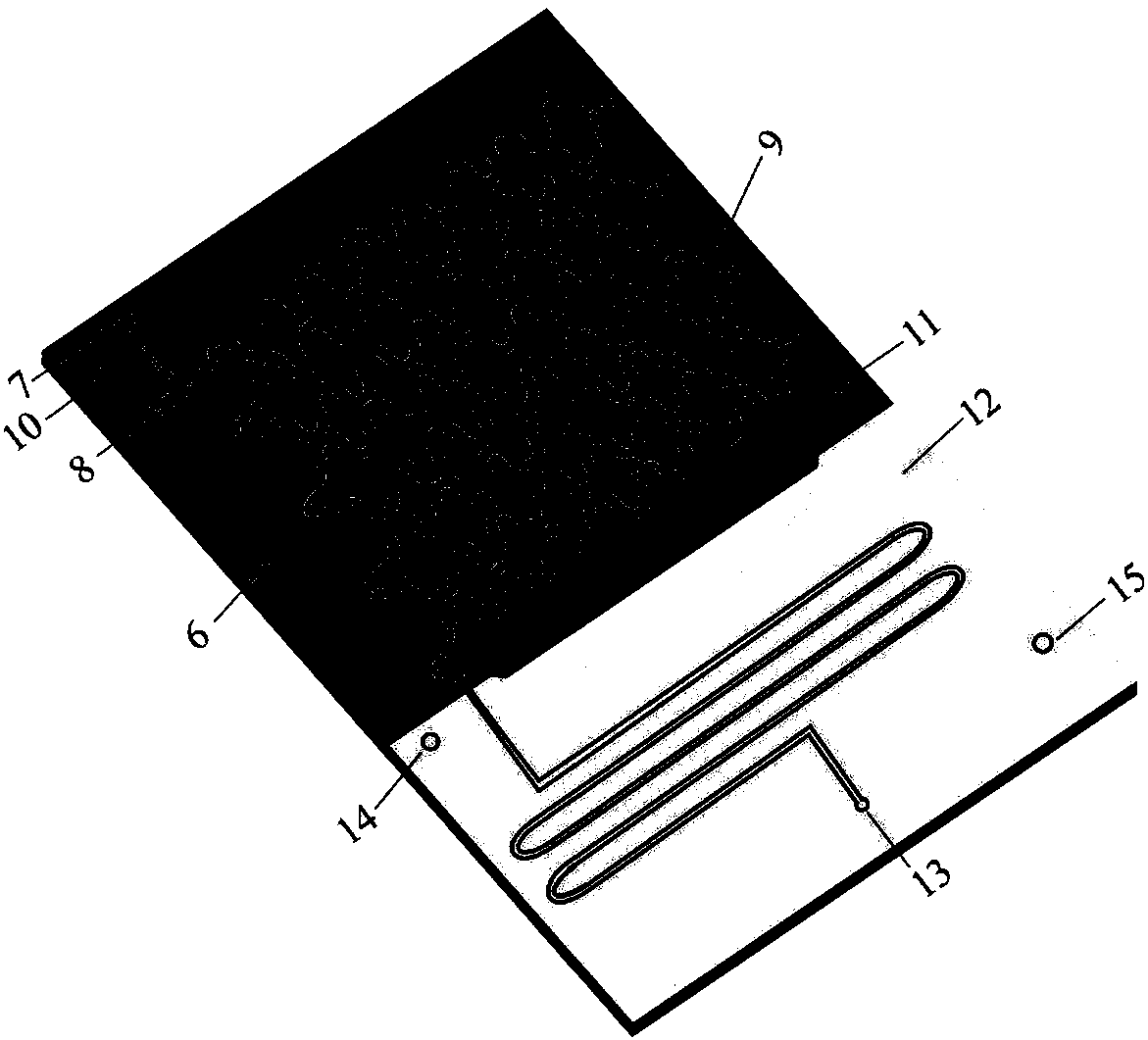
[0019] After dissolving 3.3g hydroquinone and 1.37g 6-chloro-1-hexanol with ethanol, the volume was adjusted to 100ml to make mixed raw material 1, and then 3mol / L NaOH aqueous solution 2 was prepared. The flow rate of min is transported to the heating zone 6 in the microreactor (characteristic size: 0.4 mm) 4 by the metering pump 3, the temperature in the heating zone is controlled at 135°C, and the two materials enter the reactor from the reactor inlet 7 and 8 respectively after mixing , react in the microchannel 9, the material residence time is 5.4min, the material after the reaction enters the cooling zone 12, and the reaction is terminated by cold fluid heat exchange, and the product is collected at the outlet 13. The collected sample was transferred into a separatory funnel, extracted by n-hexane and then phase-separated. Unreacted 6-chloro-1-hexanol was present in the organic phase, and the aqueous phase was neutralized to pH=2 with hydrochloric acid. Ethanol is evapor...
Embodiment 2
[0021] Process is the same as example 1, only change the proportioning of reactant hydroquinone and 6-chloro-1-hexanol and the residence time of material: 5.5g hydroquinone and 1.37g 6-chloro-1-hexanol are mixed with ethanol After the alcohol is dissolved, the volume is adjusted to 100ml to make a mixed raw material, 3mol / L NaOH aqueous solution is used as a catalyst, and the residence time of the material is 1.5min. 100% conversion of 6-chloro-1-hexanol and 50% yield of 4-(6-hydroxyhexyloxy)phenol were obtained.
Embodiment 3
[0023] Process is the same as example 1, only change the concentration of reactant hydroquinone and 6-chloro-1-hexanol: After dissolving 16.52g hydroquinone and 6.831g 6-chloro-1-hexanol with ethanol, settle to 100ml is made into mixed raw material, and the NaOH aqueous solution of 3mol / L is used as catalyst, and the material residence time is 5min. The conversion rate of 6-chloro-1-hexanol was 100%, and the yield of 4-(6-hydroxyhexyloxy)phenol was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com