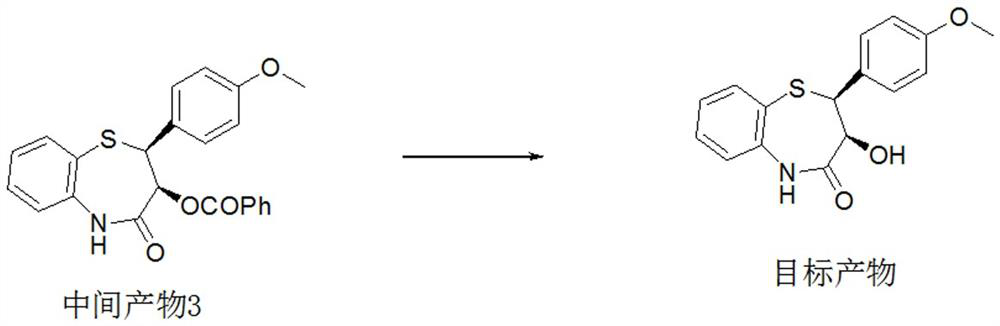
Preparation method of 2-(4-methoxyphenyl)-3-hydroxy-2, 3-dihydro-1, 5-benzothiazepine ketone
A technology of methoxyphenyl and benzothiazide, which is applied in the field of chemical pharmaceuticals, can solve the problems of high cost and achieve the effects of high yield, high product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
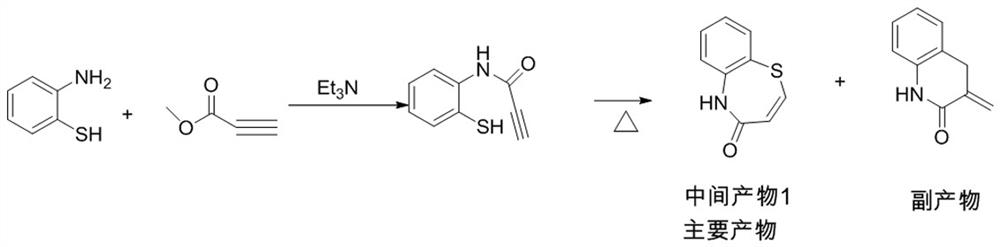
[0048] Intermediate 1:
[0049] 1000ml reaction flask, under nitrogen protection, add o-aminothiophenol (62.6g, 0.5mol, 1.0eq), toluene 500ml, triethylamine (10.1g, 0.1mol, 0.2eq), cool down to -18°C, add acetone dropwise Methyl alkynoate (46.2g, 0.55mol, 1.1eq), control the temperature at -18°C, stir well, keep warm for half an hour, until the reaction of o-aminothiophenol is complete, heat up, evaporate the low boiling point substances, and then heat up to toluene Reflux, react for 2 hours, cool, wash with water, concentrate and cool, precipitate crystals, filter with suction, and dry to obtain intermediate product 1, 69.9g, with a yield of 78.9% and a purity of 99.2%.
Embodiment 2
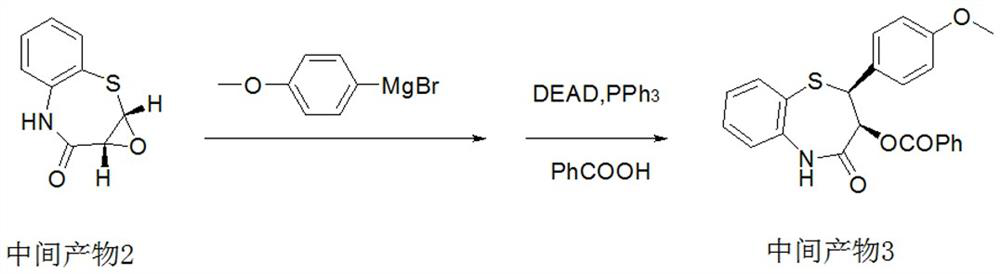
[0051] Intermediate 2:
[0052] In a 1000ml reactor, add 500ml of dichloromethane, catalyst 1 (10g, a mixture of titanium tetraisopropoxide and L-(+)-diisopropyl tartrate in a mass ratio of 1:1), and intermediate 1 (53g , 0.3mol, 1.0eq), cooled to 0°C, controlled the internal temperature to 0°C, then added dropwise tert-butanol peroxide (28.4g, 0.315mol, 1.05eq) to react for half an hour, filtered, washed with water, concentrated to obtain the intermediate product 2 (44.1g, 0.228mol), yield 76%, purity 98.5%.
Embodiment 3
[0054] In a 1000ml reaction flask, under the protection of nitrogen, add 300ml of tetrahydrofuran, 12g of magnesium chips, 0.1g of iodine, and a small amount of 4-methoxybromobenzene. -Methoxybromobenzene (39.3g, 0.21mol), refluxed for 1 hour to obtain a solution of (4-methoxyphenyl)magnesium bromide in tetrahydrofuran.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com