Method for suppressing shuttle flying of polysulfide ions in lithium-sulfur battery
A lithium-sulfur battery, polysulfur technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as large volume changes, difficult to withstand batteries, and reduced electrolyte conductivity, to inhibit dissolution and diffusion, and protect metals. Lithium, practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
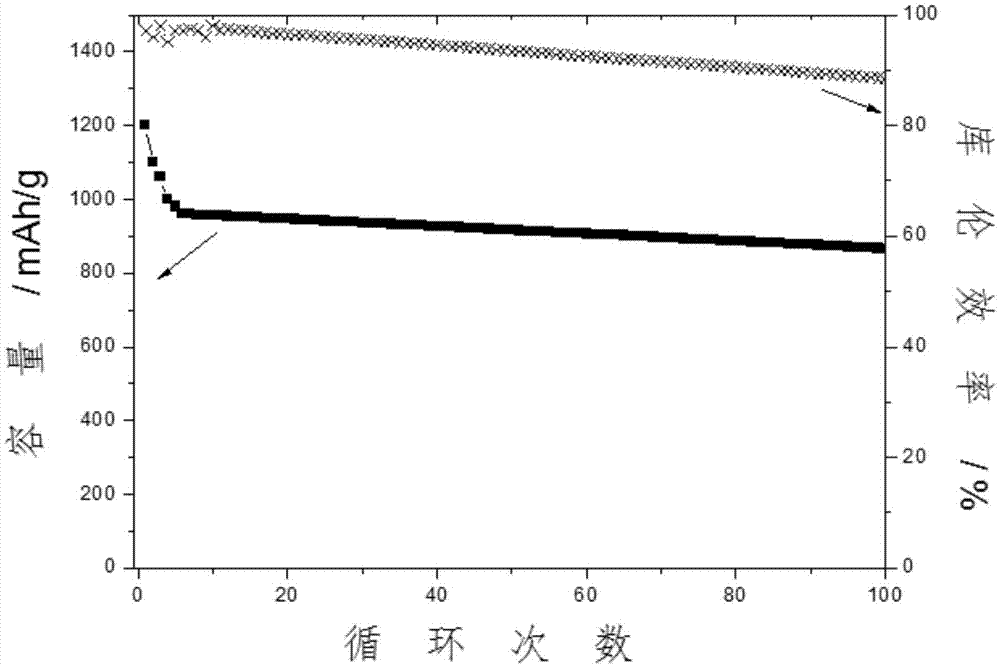
[0041] Add 0.25 g of 1-ethyl-2-methylpyridinium bromide into 10 g of 1M LiTFSI / (DME+DOL) electrolyte, and stir vigorously to obtain a clear electrolyte. The composite film obtained above is used to assemble a lithium-sulfur button battery, and its positive electrode is a carbon-sulfur compound (75% sulfur filling, PVDF binder). The battery is charged and discharged at a rate of 0.1 for 100 cycles, and the average Coulombic efficiency is 97.5% ( figure 1 );
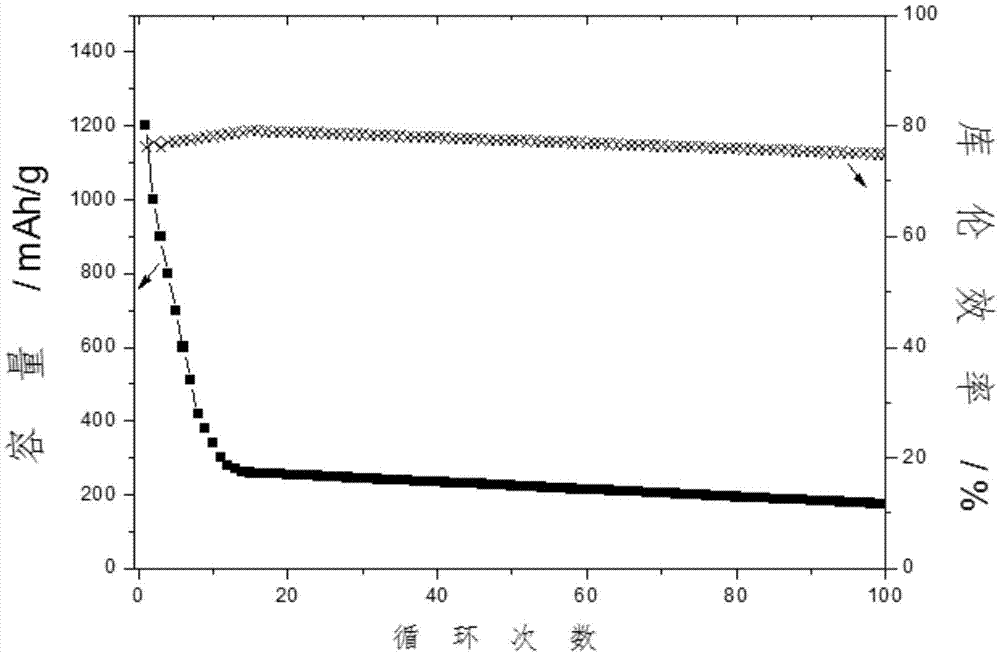
[0042] And when adopting the electrolyte solution without complexing agent, other conditions remain unchanged, the average coulombic efficiency of battery only has 68% ( figure 2 ).
Embodiment 2
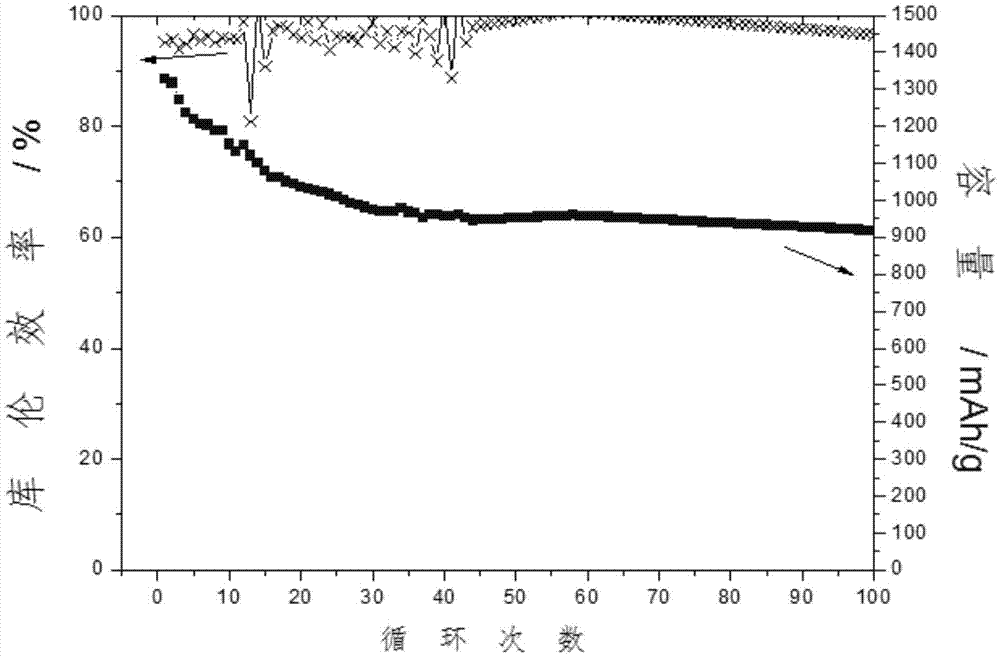
[0044] Add 0.4 g of 1-alkyl-3-alkylpyridinium halides into 10 g of 1M LiTFSI / (DME+DOL) electrolyte, and stir vigorously to obtain a clear electrolyte. A lithium-sulfur button battery was assembled using the electrolyte solution obtained above, and its positive pole was a carbon-sulfur composite (80% sulfur filling, PVDF binder). The battery was charged and discharged at a rate of 0.1 for 100 cycles, and the average Coulombic efficiency was 99% ( image 3 ).
Embodiment 3
[0046] Add 0.504 g of 1-alkyl-3-alkylimidazolium halides into 12 ml of 1M LiTFSI / (DME+DOL) electrolyte, and dissolve completely by stirring to obtain a clear electrolyte. A lithium-sulfur button battery was assembled using the electrolyte solution obtained above, and its positive electrode was a carbon-sulfur composite (58% sulfur filling, PVDF binder). The battery was charged and discharged at a rate of 0.1 for 40 cycles, and the average Coulombic efficiency was 98.7% ( Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com