Application of pan-HDAC inhibitor to preparation of drug used for treating uveal melanoma
A technology for melanoma and grape tumor, applied in drug combination, antitumor drugs, pharmaceutical formulations, etc., can solve the problems of unclear efficacy and mechanism of uveal melanoma, and achieve improved treatment effectiveness, improved quality of life, prolonged Effects on patient lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
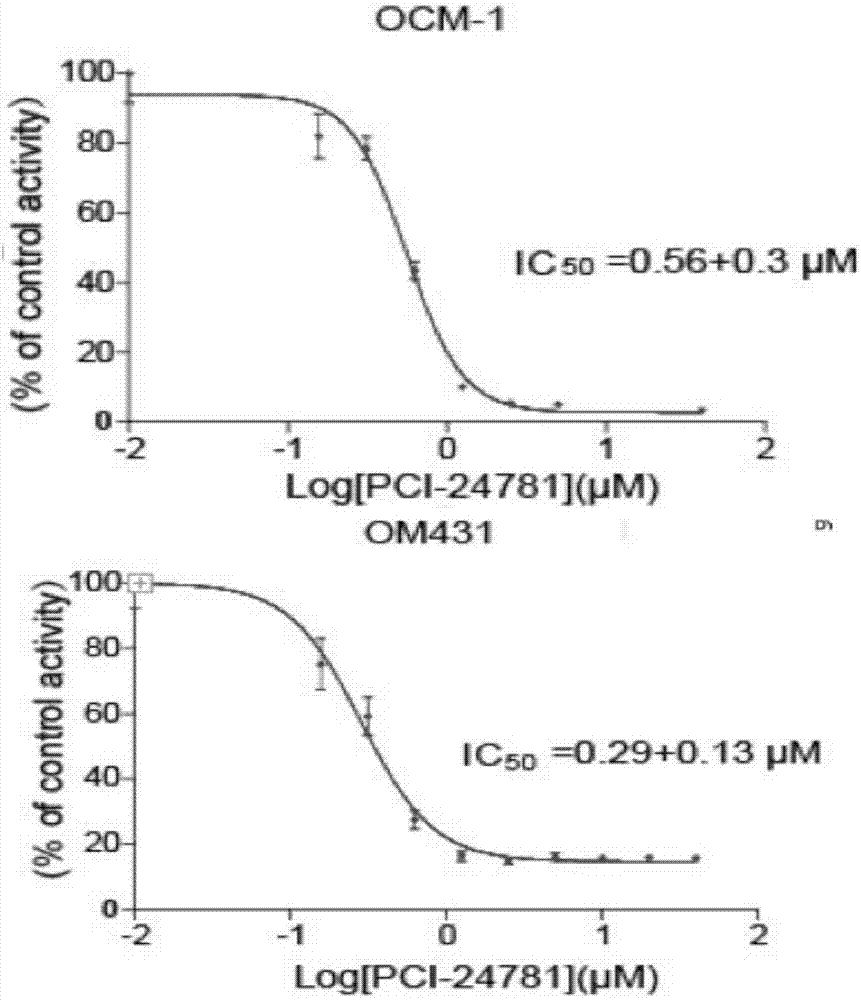
[0016] IC50 determination experiment
[0017] Experimental materials: PCI-24781 (Abexinostat) was purchased from Selleck (China), and the cell-title glo kit was purchased from Promega (USA)
[0018] Experimental procedure: OCM1 and OM431 cells were seeded in a 384-well plate, 2500 cells per well, 50ul culture medium, 10 concentration gradients were added to PCI-24781 (Abexinostat), 37 ° C, 5% CO2 After 72 hours of culture, add cell-title The glo reagent was incubated at room temperature for 10 minutes, tested on the computer, and calculated IC50 according to the measured experimental results, that is, the median lethal dose. See the experimental results figure 1 shown.
Embodiment 2
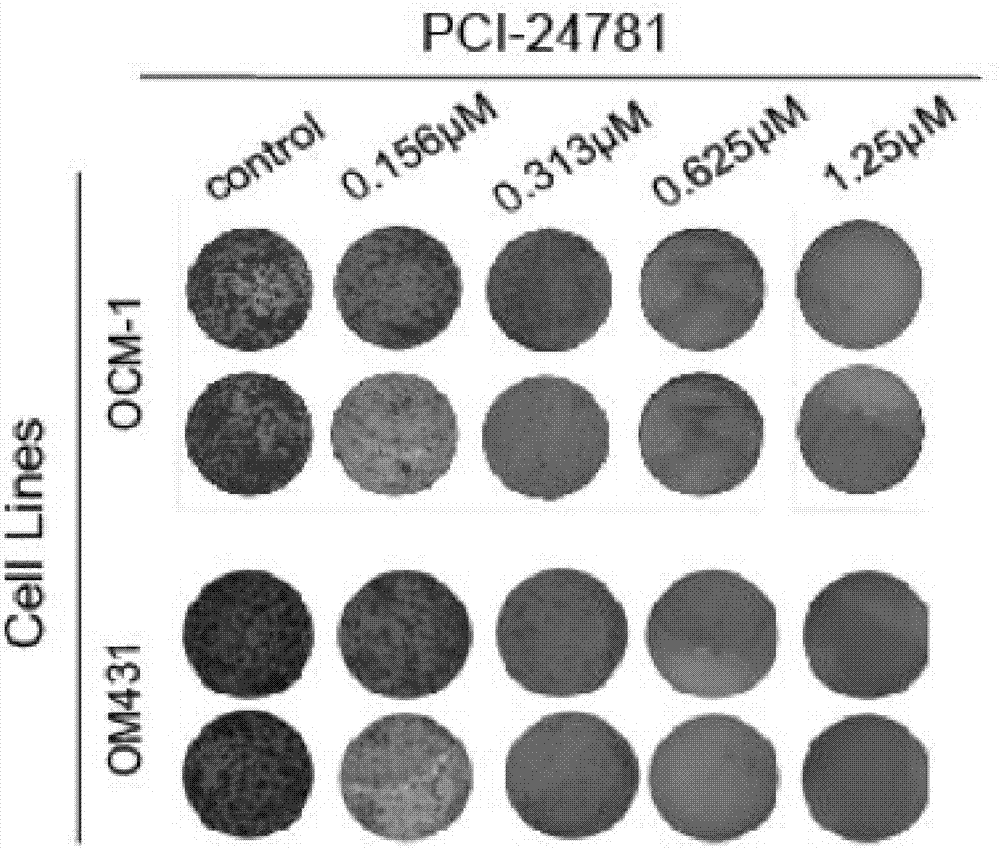
[0020] Plate cloning experiments
[0021] Experimental procedure: OCM1 and OM431 cells were seeded in a 6-well plate, 1000 cells per well, 2ml of culture medium, 5 concentration gradients were added to PCI-24781 (Abexinostat), 37 ° C, 5% CO2 After 7 days of culture, crystal violet staining, See the experimental results figure 2 shown. It can be seen that PCI-24781 (Abexinostat) inhibits the proliferation of uveal melanoma cells in a concentration-dependent manner.
Embodiment 3
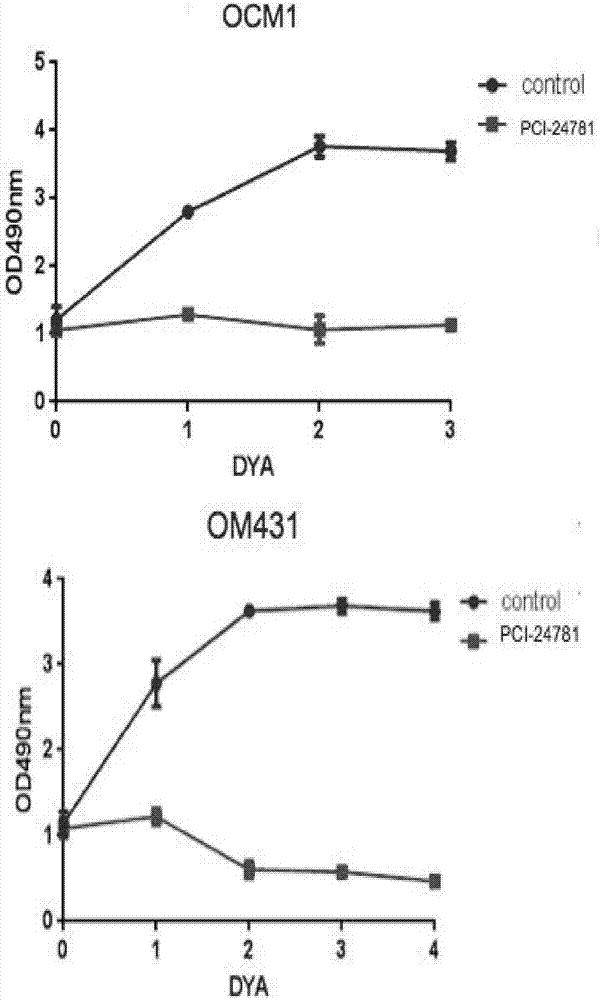
[0023] Cell Proliferation Assay (CCK8)
[0024] Experimental materials: CCK8 was purchased from Japan Tongren Chemical Co., Ltd.
[0025] OCM1 and OM431 cells were seeded in 96-well plates, 5000 cells per well, 100ul culture medium, 10umol PCI-24781 (Abexinostat), 37°C, 5% CO2 culture, 10ul CCK8 was added at 0h, 24h, 48h, and 72h respectively, and continued Incubate at 37°C for 4 hours, and measure the absorbance value on the computer. The results are as follows: image 3 shown. It can be seen that PCI-24781 (Abexinostat) can significantly inhibit the proliferation of uveal melanoma cells.
[0026] The present invention finds for the first time that PCI-24781 (Abexinostat) can significantly inhibit the growth of uveal melanoma cells, and the effect of the drug increases as the concentration increases. The invention aims to provide new therapeutic drugs for the clinical treatment of uveal melanoma, improve the effectiveness of treatment, prolong the life of patients and impr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com