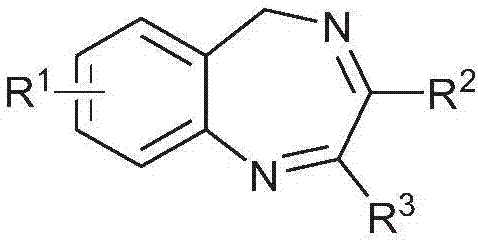
Application of 5-hydrogen-1,4-benzodiazepine compound in treatment of liver cancer
A benzodiazepine, a technology for the treatment of liver cancer, applied in medical preparations containing active ingredients, organic chemistry, drug combinations, etc., can solve problems such as less research, achieve strong inhibitory activity, and good development and application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
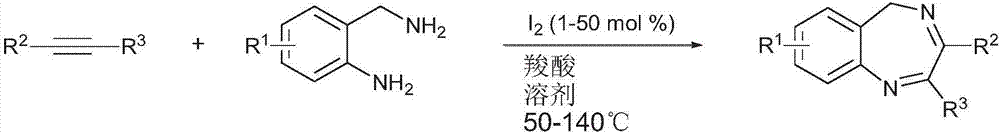
[0031] A kind of 5-hydrogen-1 of the present invention, the synthetic route of 4-benzodiazepine compound is as follows figure 2 Shown; Its concrete synthetic method comprises the following steps:
[0032] (1) In the reaction device, sequentially add 1 to 50 mol% iodine elemental catalyst, 1,2-disubstituted acetylene compound and organic solvent, and react under heating at 50 to 140°C;
[0033] (2) After reacting for a period of time, add 2-aminobenzylamine compound and acidic compound successively, continue heating reaction;
[0034] (3) After the reaction is complete, the 5-hydrogen-1,4-benzodiazepines are obtained through conventional post-treatment.
[0035] In step (1), the reaction temperature can be selected to be 80-140°C, and generally 130°C is more selected during the reaction process; in step (2), the reaction temperature can be selected to be 80-120°C, generally during the reaction The process would be more preferably 100°C.
Embodiment 1
[0039] The synthesis of 2,3-diphenyl-5-hydrogen-1,4-benzodiazepine (compound 1), its structural formula is:
[0040]
[0041] Into the reaction tube, add 10.1 mg of iodine, 35.6 mg of 1,2-toluene, and 2 mL of dimethyl sulfoxide in sequence, react in an oil bath at 130°C for 24 hours, cool the reaction solution to room temperature, and then add 97.7 mg of 2-aminobenzylamine and 1 mL of acetic acid were reacted in an oil bath at 100°C for 20 hours, and finally 51.6 mg of compound 1 was obtained by conventional post-treatment, with a yield of 87%.
[0042] Compound 1 has a melting point of 131-132°C.
[0043] The NMR characterization results of compound 1 are: 1 H NMR (400MHz, CDCl 3 ): δ 7.88-7.77(m, 2H), 7.57-7.47(m, 3H), 7.42-7.30(m, 5H), 7.21(t, J=7.0Hz, 4H), 4.86(d, J=10.6Hz , 1H), 3.97(d, J=10.6Hz, 1H); 13 C NMR (100MHz, CDCl 3 )δ 54.8, 125.7, 126.8, 128.0, 128.3, 128.4, 128.5, 128.6, 129.9, 130.7, 130.8, 137.4, 137.8, 147.5, 160.5, 160.6.
Embodiment 2
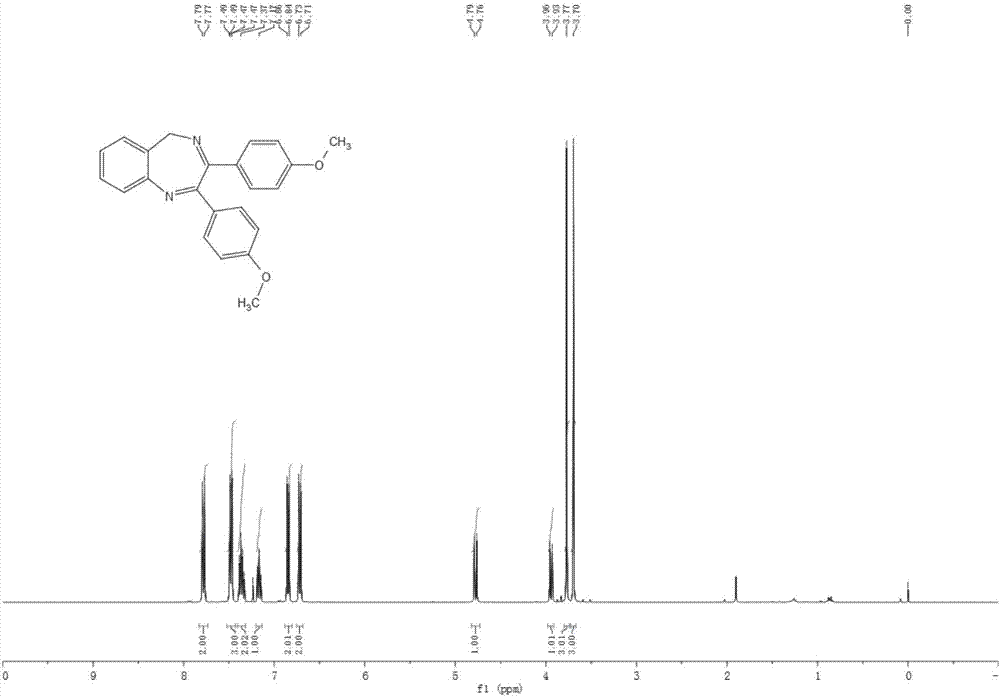
[0045] The synthesis of 2,3-bis(4-methylphenyl)-5-hydrogen-1,4-benzodiazepine (compound 2), its structural formula is:
[0046]
[0047] Into the reaction tube, add 10.1 mg of iodine, 41.2 mg of 1,2-di(4-methylphenyl)acetylene, and 2 mL of dimethyl sulfoxide in sequence, react in an oil bath at 130°C for 24 hours, and cool the reaction solution to At room temperature, 97.7mg of 2-aminobenzylamine and 1mL of acetic acid were added in sequence, and placed in an oil bath at 100°C for 20 hours. Finally, 48.0mg of compound 2 was obtained by conventional post-treatment, with a yield of 74%.
[0048] Compound 2 has a melting point of 150-152°C.
[0049] The NMR characterization results of compound 2 are: 1 H NMR (400MHz, CDCl 3 ): δ 7.72(d, J=8.0Hz, 2H), 7.51(d, J=7.7Hz, 1H), 7.45-7.30(m, 4H), 7.21-7.09(m, 3H), 7.00(d, J =7.8Hz, 2H), 4.81(d, J=10.6Hz, 1H), 3.95(d, J=10.6Hz, 1H), 2.31(s, 3H), 2.22(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ 21.4, 21.5, 54.7, 125.6, 126.5, 127.9, 128.3, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com