Protein markers assisting diagnosis of severe secondary pulmonary tuberculosis
A technology for auxiliary diagnosis and pulmonary tuberculosis, applied in biological testing, material inspection products, etc., can solve problems such as aggravating protein markers, aggravating pathophysiological mechanisms, and lack of progression of pulmonary tuberculosis lesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
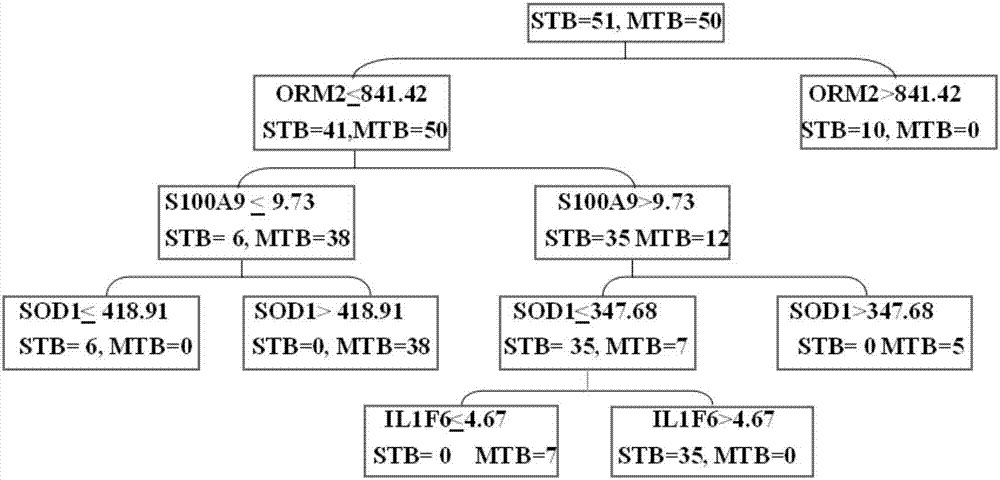
[0077] Example 1, Screening of protein markers
[0078] 1. Research object
[0079] The peripheral blood plasma samples of 6 patients diagnosed with secondary pulmonary tuberculosis from March 2015 to December 2015 in Beijing Chest Hospital Affiliated to Capital Medical University were collected. Among them, there were 3 cases of severe secondary pulmonary tuberculosis, 2 males and 1 female, with an average age of 51.33±16.66 years. There were 3 cases of mild secondary pulmonary tuberculosis, 2 males and 1 female, with an average age of 52.67±22.82 years. The informed consent protocol and research procedures were approved by the ethics committee, and all patients and healthy volunteers who agreed to participate in this study signed an informed consent form.
[0080] 2. Screening protein markers
[0081] The peripheral blood plasma samples of the research subjects were taken, proteins were extracted, and the differentially expressed proteins were screened for the severe seco...
Embodiment 2
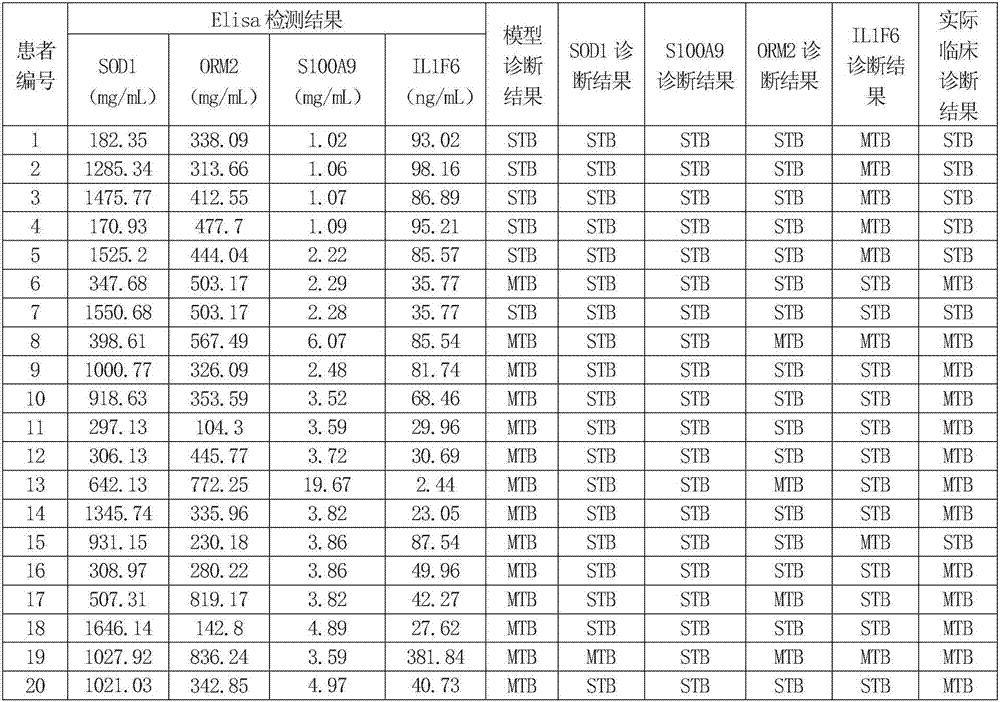
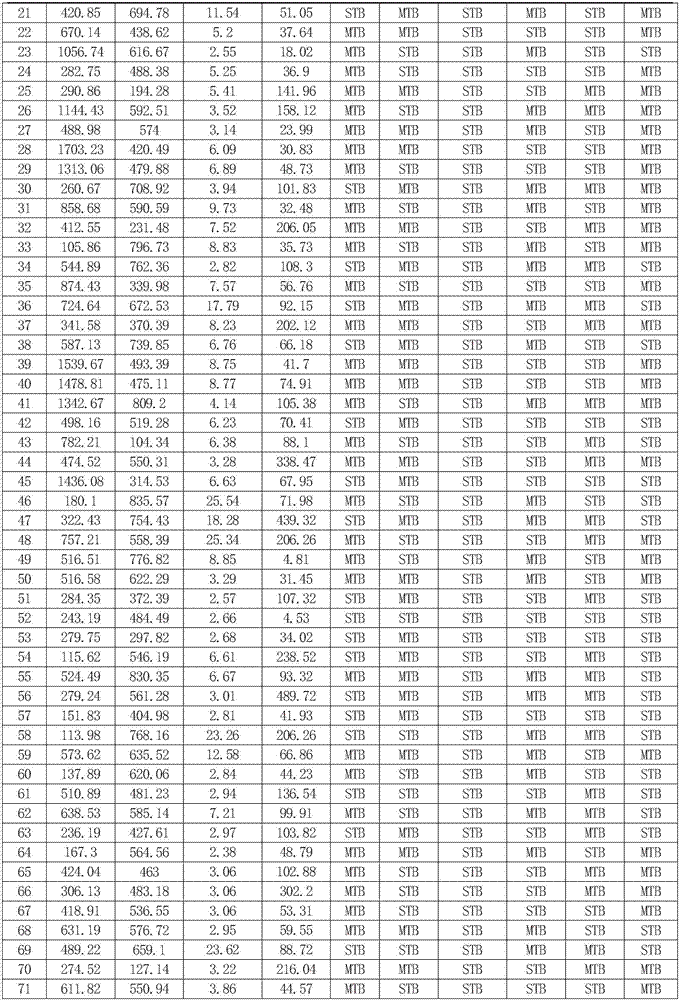
[0086] Embodiment 2, establishment and verification of diagnostic model
[0087] 1. Selection and grouping of research objects
[0088] Collected peripheral blood plasma samples from 143 cases of patients diagnosed with secondary pulmonary tuberculosis in Beijing Chest Hospital Affiliated to Capital Medical University from March 2015 to December 2015. The patients were divided into severe secondary pulmonary tuberculosis group with 72 cases and mild secondary There were 71 cases in the pulmonary tuberculosis group. The informed consent protocol and research procedures were approved by the ethics committee, and all patients and healthy volunteers who agreed to participate in this study signed an informed consent form.
[0089] 2. Elisa detection
[0090] The peripheral blood plasma samples collected in step 1 were taken, and the concentrations of SOD1, S100A9, ORM2, and IL1F6 in plasma were detected using SOD1 Elisa kit, S100A9 Elisa kit, ORM2 Elisa kit, and IL1F6 Elisa kit, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com