Preparation method of galanthamine key intermediate
A technology of galantamine and intermediates, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., can solve the problems of low industrialized production yield of galantamine, etc., and achieves control of production costs, Simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
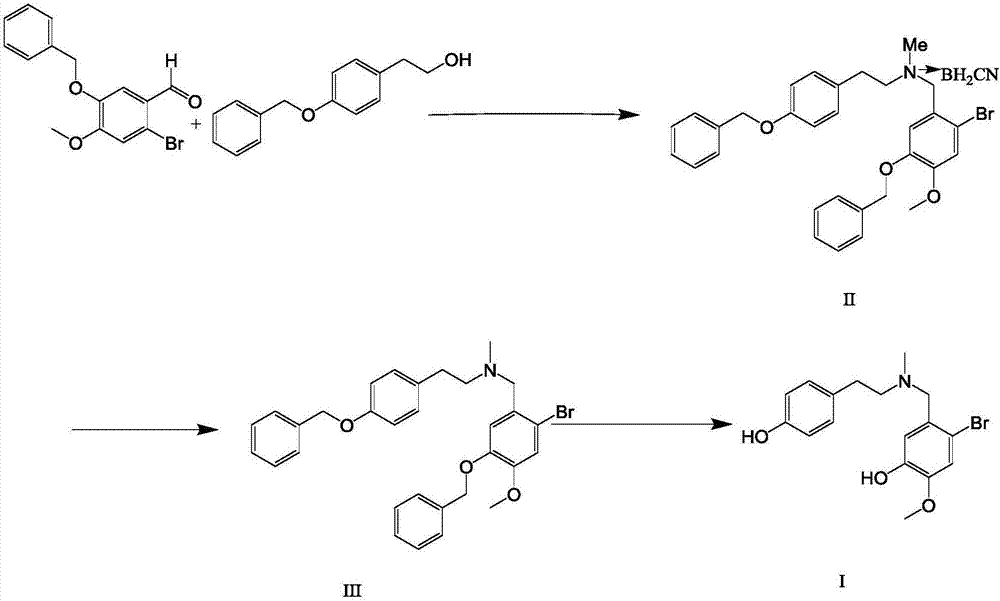
[0022] (1) Preparation of a dichloromethane solution of N-methyl-(4-benzyloxyphenethyl)-2-bromo-5-benzyloxy-4-methoxybenzylamine cyanoborohydride
[0023] 24g of p-benzyloxyphenethyl alcohol, 35g of 2-bromo-5-benzyloxy-4-methoxybenzaldehyde and 10g of methylamine hydrochloride were dispersed and dissolved in 300ml of 1,4-dioxane, and 30g of cyanoborohydrogenation Dissolve sodium in 10ml acetic acid, drop it into the raw material solution at room temperature, react at room temperature, monitor the reaction by HPLC until the basic reaction of the raw material is complete, add water to quench the reaction, extract with 500ml dichloromethane and 200ml water, obtain the organic phase, and obtain N- A dichloromethane solution of methyl-(4-benzyloxyphenethyl)-2-bromo-5-benzyloxy-4-methoxybenzylamine cyanoborohydride, and N- The purity of methyl-(4-benzyloxyphenethyl)-2-bromo-5-benzyloxy-4-methoxybenzylamine cyanoborohydride was 95.3%.
[0024] (2) Preparation of N-methyl-(4-benzylox...
Embodiment 2
[0029] (1) Preparation of a dichloromethane solution of N-methyl-(4-benzyloxyphenethyl)-2-bromo-5-benzyloxy-4-methoxybenzylamine cyanoborohydride
[0030] 24g p-benzyloxyphenethyl alcohol, 35g 2-bromo-5-benzyloxy-4-methoxybenzaldehyde and 10g methylamine hydrochloride are dispersed and dissolved in 300ml tetrahydrofuran, 30g sodium cyanoborohydride is dissolved in 10ml acetic acid, Drop into the raw material solution at room temperature, react at room temperature, monitor the reaction by HPLC until the basic reaction of the raw material is complete, add water to quench the reaction, extract with 500ml dichloromethane and 200ml water, obtain the organic phase, and obtain N-methyl-(4-benzyl Oxyphenethyl)-2-bromo-5-benzyloxy-4-methoxybenzylamine cyanoborohydride in dichloromethane solution, HPLC measured N-methyl-(4-benzyl The purity of oxyphenethyl)-2-bromo-5-benzyloxy-4-methoxybenzylamine cyanoborohydride was 95.1%.
[0031] (2) Preparation of N-methyl-(4-benzyloxyphenethyl)-2...
Embodiment 3
[0036] (1) Preparation of a dichloromethane solution of N-methyl-(4-benzyloxyphenethyl)-2-bromo-5-benzyloxy-4-methoxybenzylamine cyanoborohydride
[0037] 24g p-benzyloxyphenethyl alcohol, 35g 2-bromo-5-benzyloxy-4-methoxybenzaldehyde and 10g methylamine hydrochloride are dispersed and dissolved in 300ml tetrahydropyran, 30g sodium cyanoborohydride is dissolved in 10ml In acetic acid, drop into the raw material solution at room temperature, react at room temperature, monitor the reaction by HPLC until the basic reaction of the raw material is complete, add water to quench the reaction, extract with 500ml dichloromethane and 200ml water, obtain the organic phase, and obtain N-methyl-( 4-benzyloxyphenethyl)-2-bromo-5-benzyloxy-4-methoxybenzylamine cyanoborohydride dichloromethane solution, HPLC records the solution N-methyl-( The purity of 4-benzyloxyphenethyl)-2-bromo-5-benzyloxy-4-methoxybenzylamine cyanoborohydride was 95.3%.
[0038] (2) Preparation of N-methyl-(4-benzyloxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com