Pharmaceutical composition for preventing or treating gleevec-resistant leukemia, containing, as active ingredient, ginsenoside f1 or rg3
一种人参皂苷、格列卫耐的技术,应用在含有效成分的医用配制品、碳水化合物有效成分、药物组合等方向,能够解决格列卫耐药性白血病治疗效果差、需要长期给药等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
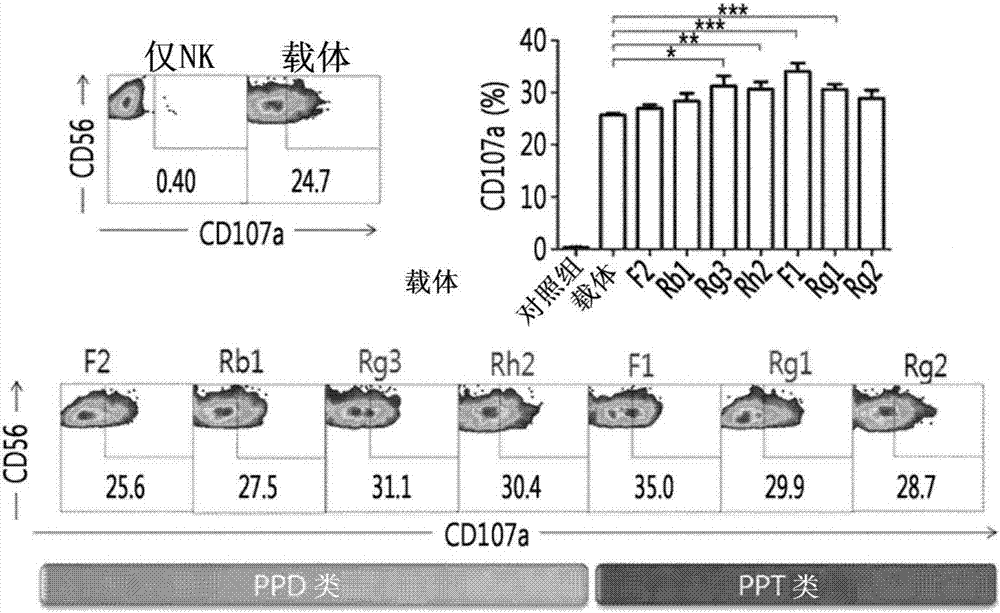
[0053] Example 1: Discovery of ginsenosides that enhance the cytotoxic activity of NK cells
[0054] In order to discover ginsenosides that affect the cytotoxic activity of NK cells, 15 kinds (C-K, F2, PPD, Rb1, Rb2, Rc, Rd, Rg3, Rh2, F1, PPT, Re, Rg1, Rg2 and Rh1) were used PBMC (peripheral blood mononuclear cell, peripheral blood mononuclear cell) were treated with ginsenoside, and the level of CD107a, which is a marker protein exhibiting a cell-killing effect expressed by NK cells, was measured by flow cytometry ( figure 1 ).
[0055] At this time, the PBMCs screened in the following manner were used. That is, the extracted blood was put into a mononuclear cell preparation tube (Vacutainer Cell Preparation Tube) (sodium heparin (sodium heparin); BD Biosciences (BD biotechnology)), and centrifuged to recover buffy coat (buffy coat). ; lymphocytes and monocyte band (lymphocyte and monocyte band)), and after washing the recovered buffy coat with PBS (phosphate buffered sa...
Embodiment 2
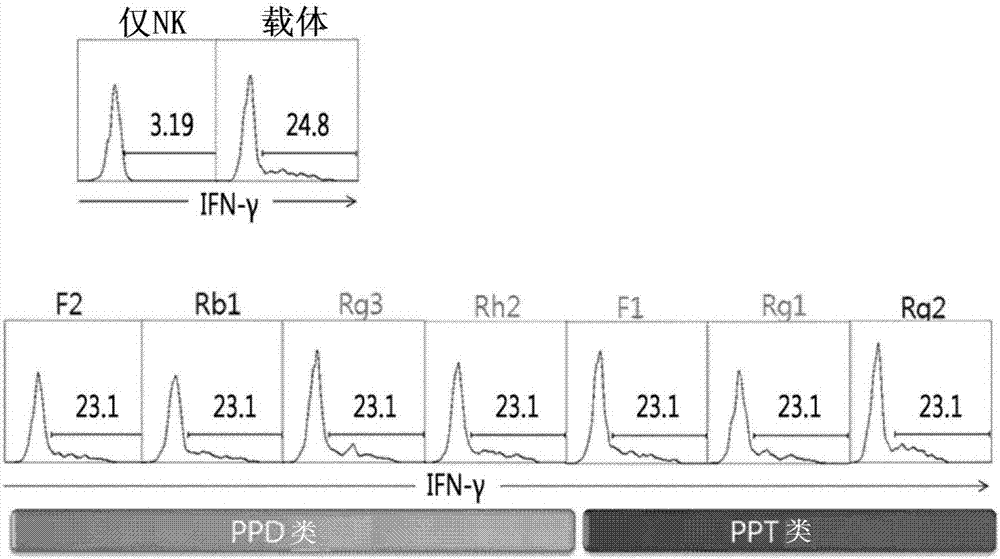
[0058] Example 2: Screen out the ginsenosides that improve the expression of the immune activity factor (IFN-γ) of NK cells
[0059] Treat with the seven ginsenosides discovered in Example 1 above, and screen out the ginsenosides that increase the expression level of the immune activity factor (IFN-γ) of NK cells ( figure 2 ). At this time, the expression level of IFN-γ was determined in the following manner. That is, the PBMCs isolated from the above-mentioned Example 1 were treated with the respective ginsenosides, and the target cells (KCL-22 cell variants) with the same number of cells were treated to stimulate, and then treated with brefeldin A (Brefeldin A) (GolgiPlug; BD Biosciences) for processing. Next, NK cells derived from PBMC were isolated by performing flow cytometric analysis using anti-CD3 antibody and anti-CD56 antibody, and the isolated NK cells were treated with Cytofix / Cytoperm (BD Biosicences) to perforate, and then Anti-IFN-γ antibody was immunostai...
Embodiment 3
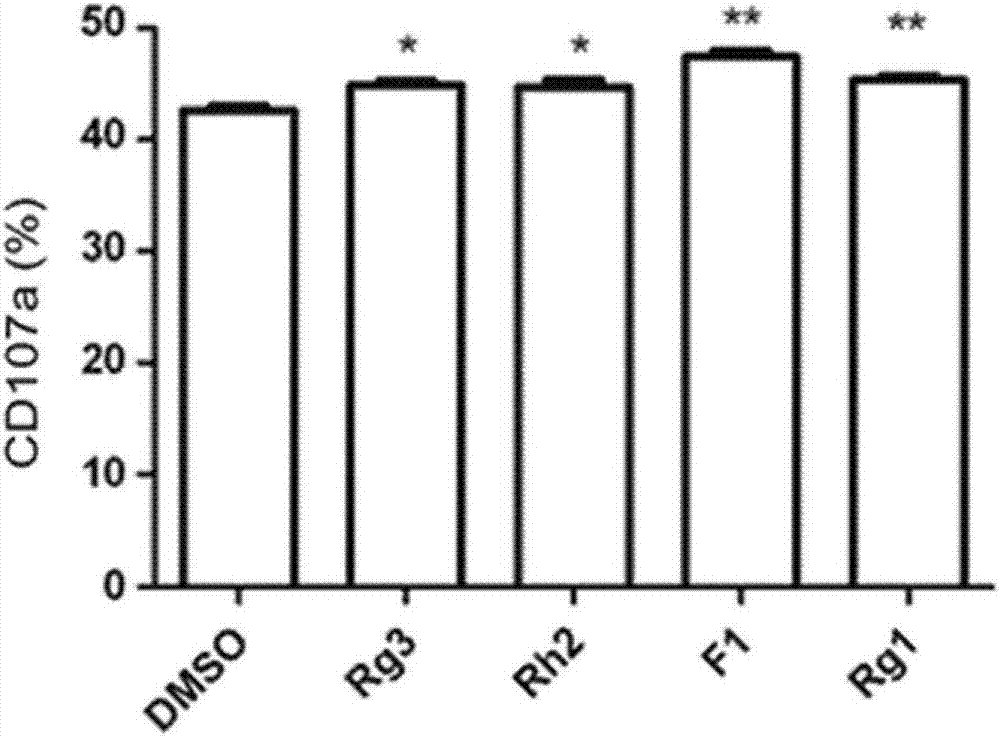
[0061] Example 3: Screening out ginsenosides that increase the level of CD107a in non-activated NK cells
[0062] From the four ginsenosides (Rg3, Rh2, F1 and Rg1) screened in Example 2 above, a ginsenoside that induces an increase in the level of CD107a in non-activated NK cells was screened.
[0063] Specifically, after treating NK cells isolated from PBMCs with the four ginsenosides to activate the NK cells, and adding a variant of KCL-22 cells as Gleevec-resistant leukemia cells, CD107a was measured and compared s level( image 3 ). At this time, as a control group, NK cells treated with DMSO (dimethyl sulfoxide) instead of ginsenoside were used.
[0064] image 3 It is a graph showing the effect of ginsenosides on increasing the expression level of CD107a by reacting a variant of KCL-22 cells, which are Gleevec-resistant leukemia cells, with NK cells. Such as image 3 As shown, it was confirmed that the level of CD107a was significantly increased when the KCL-22 ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com