Method for detecting biological activity of recombinant human interleukin-1 receptor antagonist eye drops
A technology of interleukin and receptor antagonist, applied in the field of detection of biological activity of recombinant human interleukin 1 receptor antagonist eye drops, can solve the problems of many detection steps, poor specificity, unstable results, etc. Achieve the effect of good repeatability, simple operation and easy observation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
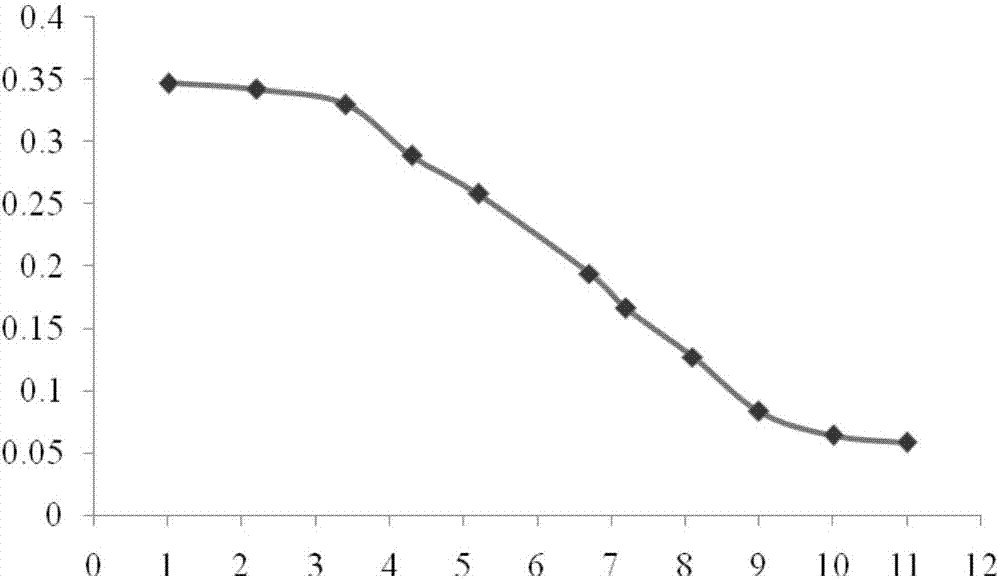
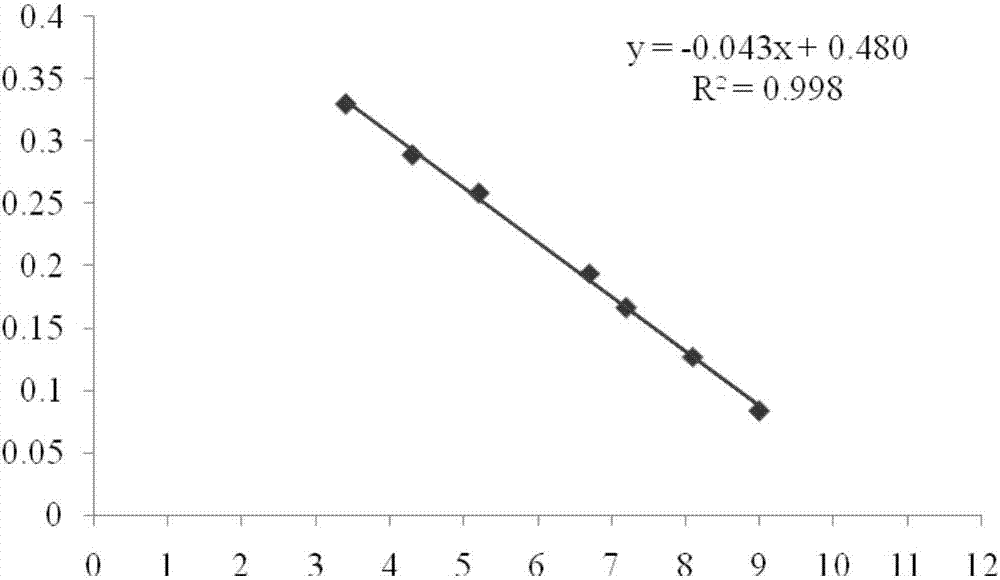
[0051] Preparation of standard solution: Dissolve 10 μg of rhIL-1ra standard (1U / mg, NIBSC) in 1.25ml of water, aliquot and freeze, and use culture medium B before use 2 Dilute 10 times to make the concentration 800ng / ml. In a 96-well culture plate, make a 3-fold serial dilution, a total of 11 dilutions, make 3 wells for each dilution, leave 50μl standard solution in each well, discard excess solution in the well. The above operations were carried out under sterile conditions.
[0052] Preparation of the test solution: get the rhIL-1ra eye drops prepared above, add culture solution B 2 , until the concentration of rhIL-1ra is 800ng / ml, in a 96-well culture plate, make 3-fold serial dilutions, a total of 11 dilutions, each dilution is made into 3 holes, and 50 μl of the test solution is left in each hole, discarded excess solution in the well. The above operations were carried out under sterile conditions.
[0053] Assay:
[0054] Add the A375-s2 cell suspension to each we...
Embodiment 2
[0065] Preparation of standard solution: Dissolve 10 μg of rhIL-1ra standard (1U / mg, NIBSC) in 1.25ml of water, aliquot and freeze, and use culture medium B before use 2 Dilute 10 times to make the concentration 800ng / ml. In a 96-well culture plate, make a 3-fold serial dilution, a total of 11 dilutions, make 2 wells for each dilution, leave 50μl standard solution in each well, discard excess solution in the well. The above operations were carried out under sterile conditions.
[0066] Preparation of the test solution: get the rhIL-1ra eye drops prepared above, add culture solution B 2 , until the concentration of rhIL-1ra is 800ng / ml, in a 96-well culture plate, make 3-fold serial dilutions, a total of 11 dilutions, each dilution is made into 2 wells, and 50 μl of the test solution is left in each well, discarded excess solution in the well. The above operations were carried out under sterile conditions.
[0067] Assay:
[0068] Add the A375-s2 cell suspension to each we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com