Macropinocytosis of human anti-CD46 antibody and targeted cancer therapy
An antibody and human technology, applied in the direction of antibodies, chemical instruments and methods, anti-animal/human immunoglobulin, etc., can solve the problem of not providing cancer cell specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0654] The following examples are offered to illustrate, not limit, the invention.
example 1
[0656] Novel anti-CD46 antibodies and uses thereof
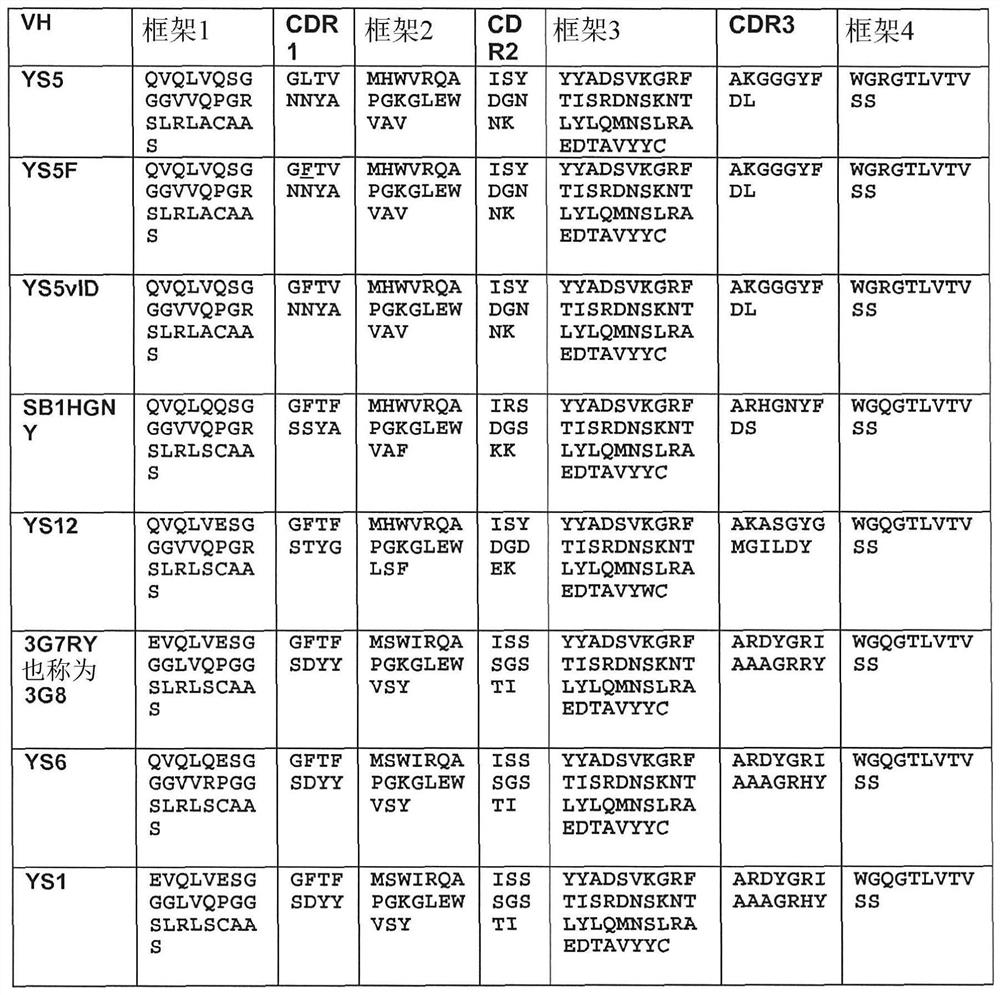
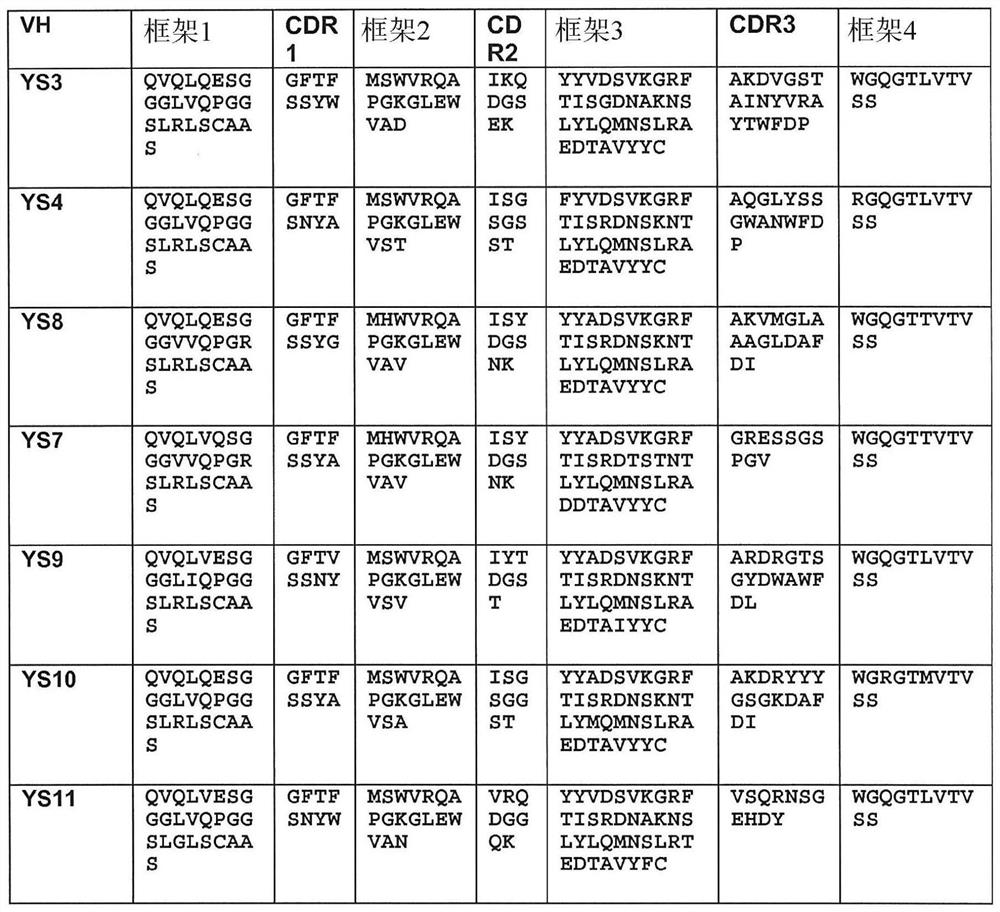
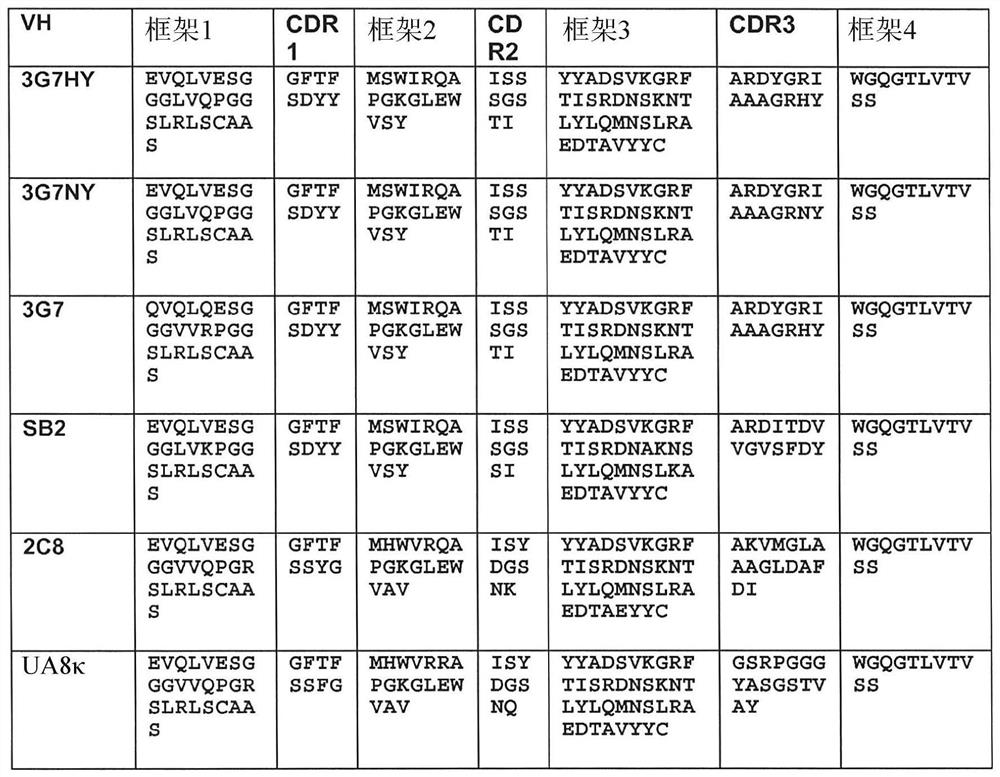
[0657] To identify novel anti-human CD46 antibodies, a recombinant Fc fusion protein consisting of Sushi domains 1 and 2 of human CD46 was formed. Since the complementary sequence elements primarily bind to domains 3 and 4, the selection of domains 1 and 2 minimizes the selection of antibodies that might strongly interfere with standard complementary sequence function. This CD46-Fc fusion was made and purified from transfected HEK293 cells by protein A affinity chromatography. For human antibody selection, pooled cDNA from peripheral blood mononuclear cells of 426 healthy human donors was used to form 5 x 10 9 A phagemid display library of 2 members was selected, and the library was selected for recombinant CD46-Fc fusion proteins. After three rounds of selection, binding phagemids were screened by FACS and sequencing. Meanwhile, an alternative strategy was employed, which involved first selection of the library on live...
example 2
[0680] CD46ADC is highly active in an intrafemoral mCRPC xenograft model.
[0681] Because more than 95% of prostate cancers metastasize to bone sites, we further investigated the efficacy of our anti-CD46 ADCs in bone xenograft models. We injected the metastatic castration-resistant prostate cancer (mCRPC) cell line LNCaP C4-2B carrying a firefly luciferase reporter into the femur of NSG mice to create an intraskeletal mCRPC xenograft model. Seven days after transplantation, CD46 ADC (YS5-mcvcpab-MMAF) was injected every 4 days for a total of 4 times. Tumor status was monitored by bioluminescent imaging during and after treatment. Such as Figure 32 As shown in , CD46ADC-treated mice showed profound tumor suppression after the treatment period and continued until the end of the experiment (day 65), indicating that our CD46ADC is highly effective in this endogenous mCRPC xenograft model.
[0682] CD46 is highly expressed in CRPC and mCRPC tissues.
[0683] In addition ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com