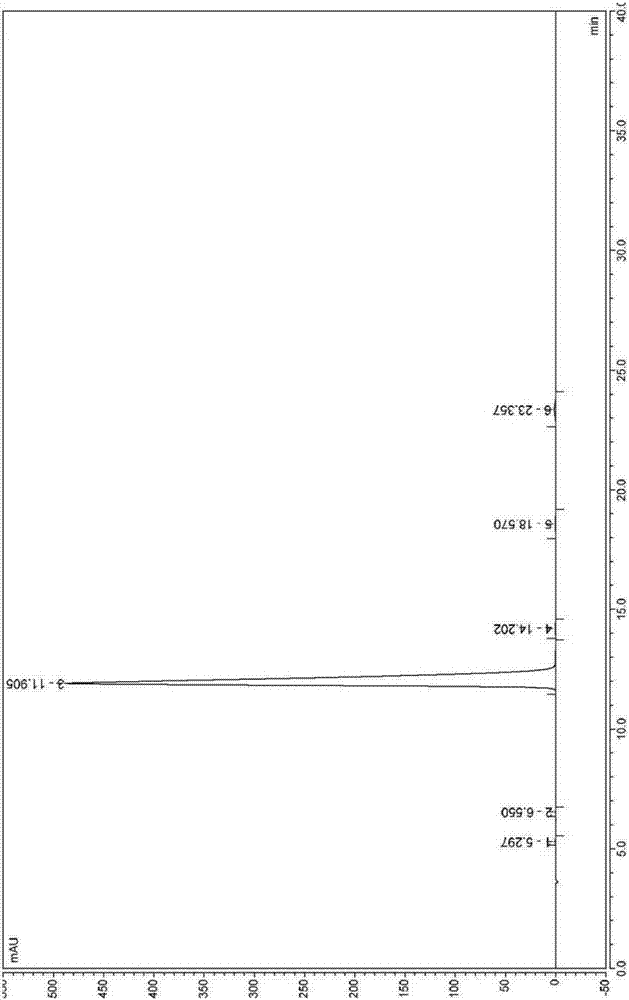
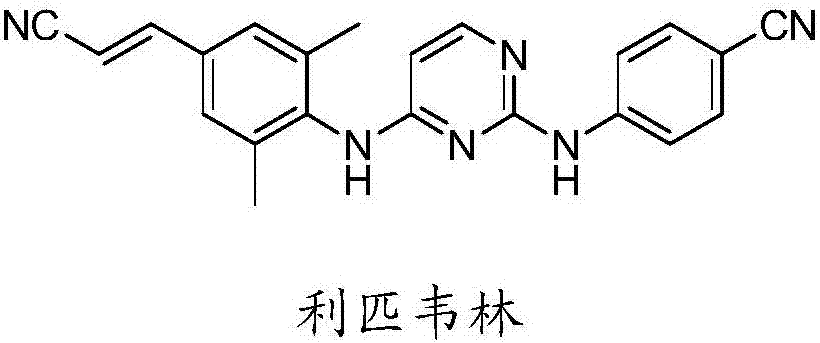
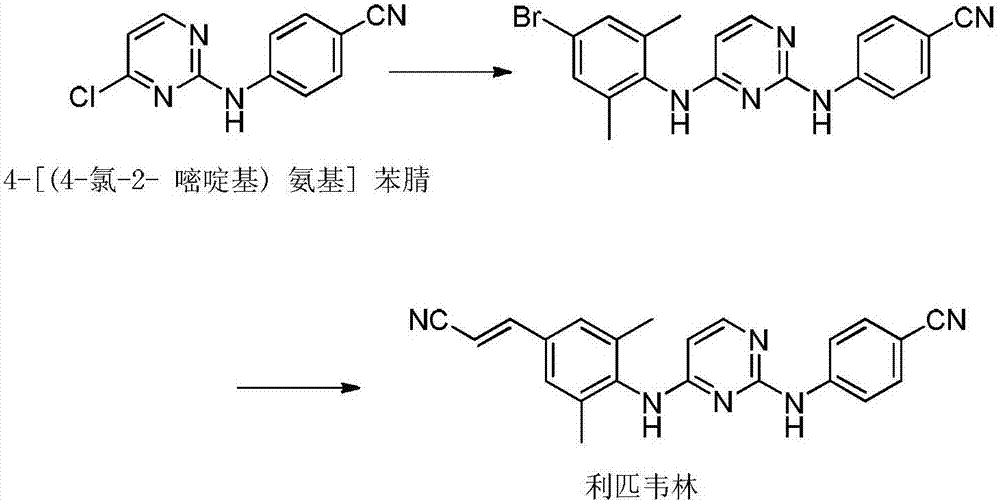
Industrial synthesis method of rilpivirine and intermediate compound
A synthesis method and technology of rilpivirine, applied in the field of medicinal chemistry synthesis, can solve the problems of long route, high temperature, unsatisfactory repetition and the like, and achieve the effects of high yield, low production cost and reduced industrialization cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1, the synthesis of formula II compound
[0040] Dilute 1000 g of ethyl formyl acetate (the compound of formula V in which R is ethyl) with 5 L of methanol, lower the temperature, slowly drop into 510 g of thiomethylisothiourea in 3 L of ethanol, and react at 5°C for 3 h. Concentrate to dryness under reduced pressure, dissolve the residue with hot water, acidify to pH = 4 with acetic acid, precipitate solid after cooling, filter, wash the filter cake with water, and recrystallize with water to obtain 764 g of beige needle-like crystals, yield: 94.9%, that is Compound of formula II;
[0041] 2, the synthesis of formula III compound
[0042] Add 1kg of compound of formula II and 692g of p-aminocyanide into 8L of pyridine, heat to 110°C, react for 8h, cool down to 0-5°C, stir and crystallize for 10h, filter, wash with methanol to obtain 1206g of light yellow solid, yield , 97%, the compound of formula III;
[0043] 3, the synthesis of formula I compound
[0044]Add 1kg...
Embodiment 2
[0065] 1, the synthesis of formula II compound
[0066] Dilute 1000g of methyl formylacetate (compound of formula V in which R is methyl) with 5L of ethanol, lower the temperature, slowly drop into 2.5L of methanol solution of 510g of thiomethylisothiourea, and react at 10°C for 3-5h. Concentrate to dryness under reduced pressure, dissolve the residue with hot water, adjust the pH to 4-5 with acetic acid, precipitate a solid after cooling, filter, wash the filter cake with water, and recrystallize with water to obtain 772 g of beige needle-like crystals, yield: 96%, That is, the compound of formula II;
[0067] 2, the synthesis of formula III compound
[0068] Add 1kg of compound of formula II and 692g of p-aminobenzocyanide into 8L of triethylamine, heat to 60°C, react for 12h, cool down to 0-5°C, stir and crystallize for 15h, filter and wash with methanol to obtain 1157.3g of light yellow solid , yield, 93%, i.e. the compound of formula III;
[0069] 3, the synthesis of f...
Embodiment 3
[0074] 1, the synthesis of formula II compound
[0075] Dilute 1000g of n-propyl formyl acetate (the compound of formula V in which R is n-propyl) with 5L of isopropanol, cool down, slowly drop into 5L of isopropanol solution of 510g of thiomethylisothiourea, and react at 35°C for 5~ 8h. Concentrate under reduced pressure to dryness, dissolve the residue with hot water, adjust the pH to 4-5 with acetic acid, and precipitate a solid after cooling, filter, wash the filter cake with water, and recrystallize with water to obtain 780.5 g of beige needle-like crystals, yield: 97% , the compound of formula II;
[0076] 2, the synthesis of formula III compound
[0077] Add 1kg of the compound of formula II and 692g of p-aminocyanide into 8L of N-methylpyrrolidone, heat to 210°C, react for 3h, cool down to 0-5°C, stir and crystallize for 10h, filter and wash with methanol to obtain a light yellow solid 1107.5 g, yield, 89%, namely formula III compound;
[0078] 3, the synthesis of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com