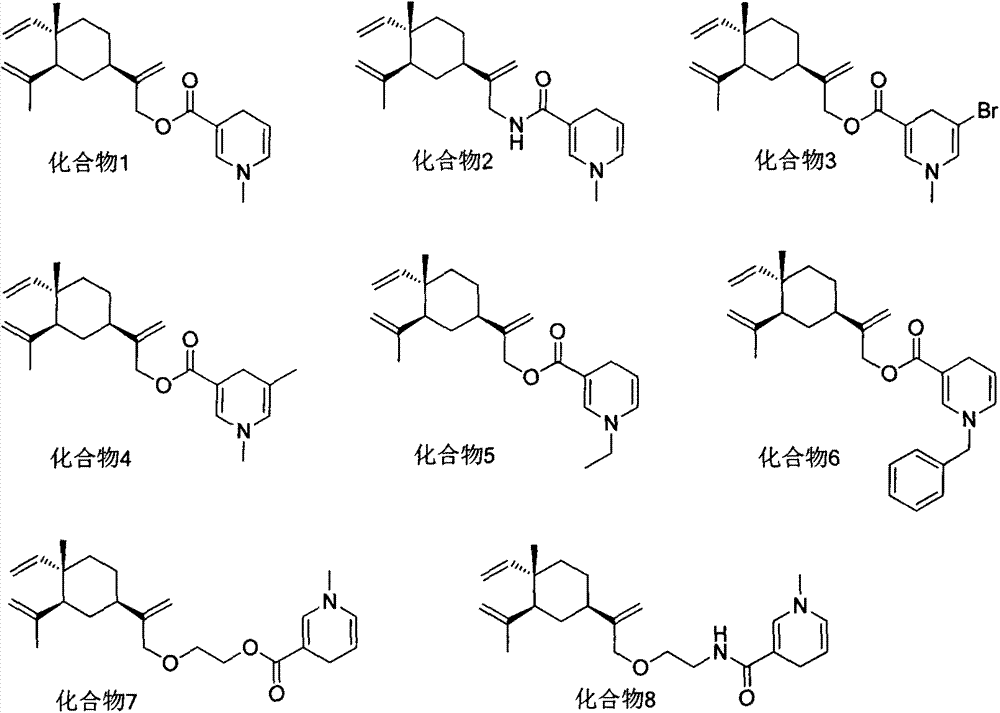
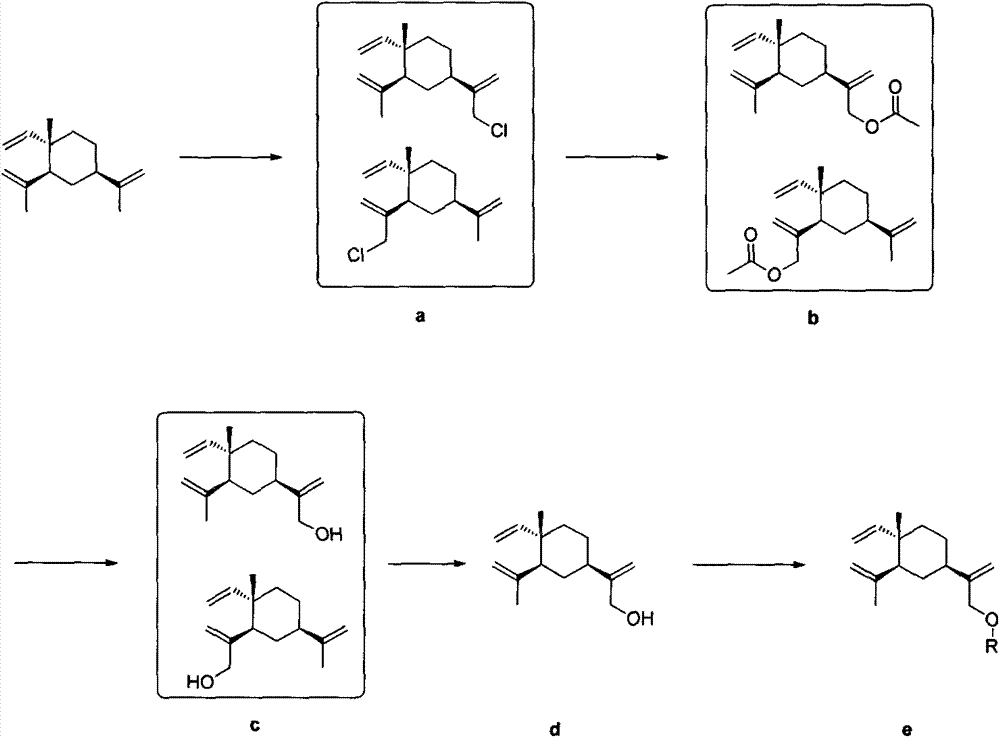
Beta-elemene derivative containing dihydropyridine structure, and preparation method and purpose thereof
A technology of dihydropyridine and elemene, applied in the field of treatment of glioma, β-elemene derivatives, β-elemene derivatives containing dihydropyridine structure, can solve low bioavailability and side effects , too strong and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 113
[0051] Example 1 Preparation of 13-beta-elemenol
[0052] β-Elemene (2.04g, 10.0mmol) was dissolved in a mixed solution of acetic acid (2mL) and dichloromethane (8mL), and slowly dropped into NaClO solution (1.6M, 11.3mL, 18.0mmol) under ice-cooling conditions , Reacted under ice bath conditions for 6h. The layers were separated, the aqueous layer was extracted with dichloromethane (3×20mL), the dichloromethane layers were combined, and saturated NaHCO 3 solution, H 2 Washed with O and saturated NaCl solution, anhydrous Na 2 SO 4 dry. After concentration, use petroleum ether column chromatography to separate β-elemene, 13-chloro-β-elemene, 14-chloro-β-elemene mixture, and 13,14-dichloro-β-elemene Alkene, the product is a colorless liquid.
[0053] β-elemene, 13-chloro-β-elemene, 14-chloro-β-elemene mixture (3.00g, containing about 13-chloro-β-elemene, 14-chloro-β-elemene Arocene (10.0 mmol) was dissolved in anhydrous DMF (15 mL), anhydrous NaOAc (2.22 g, 30.0 mmol) was ...
Embodiment 2
[0056] The preparation of embodiment 2 compound 1
[0057] Dissolve 13-β-elemenol and dihydropicolinic acid in dichloromethane, add EDCI and DMAP, stir overnight at room temperature, and perform silica gel column chromatography with petroleum ether:ethyl acetate=3:1 (V:V) to obtain Target compound 1, colorless liquid, yield 79%. 1 HNMR (CDCl 3 , 300MHz) δ: 1.04(s, 3H), 1.40-1.62(m, 6H), 1.73(s, 3H), 1.88(d, J=5.4Hz, 3H), 1.93-2.01(m, 2H), 2.84 (m, 2H), 3.42(s, 3H), 4.66(s, 2H), 4.81(s, 1H), 4.89(s, 1H), 4.91(d, J=4.3Hz, 1H), 5.01(s, 1H), 5.09(s, 1H), 5.23(m, 1H), 5.54(s, 1H), 5.87(m, 1H).
Embodiment 3
[0058] The preparation of embodiment 3 compound 2
[0059] Referring to the operation steps of Example 2, the target compound 2 was obtained as a colorless liquid with a yield of 64%. 1 H NMR (CDCl 3 , 300MHz) δ: 1.01(s, 3H), 1.40-1.61(m, 6H), 1.70(s, 3H), 1.82(d, J=5.1Hz, 3H), 1.98-2.01(m, 2H), 2.83 (m, 2H), 3.40(s, 3H), 4.69(s, 2H), 4.80(s, 1H), 4.94(s, 1H), 4.98(d, J=4.3Hz, 1H), 5.03(s, 1H), 5.09(s, 1H), 5.53(m, 1H), 5.64(s, 1H), 5.92(m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com