Method for rapidly evaluating potential teratogenicity of Chinese patent medicines by using embryo of zebrafish
A technology for zebrafish embryos and Chinese patent medicines, which can be used in the testing of pharmaceutical preparations, material inspection products, etc., can solve problems such as low practicability and no established criteria for potential teratogenicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
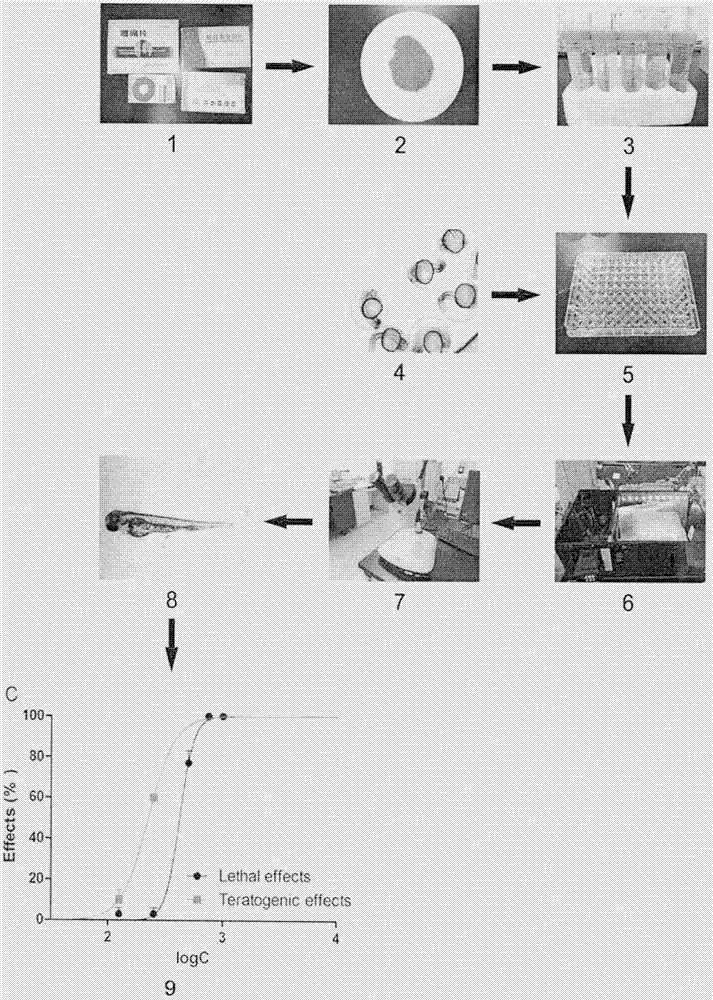
[0011] 1. Grind Fule granules into fine powder, accurately weigh 250 mg, add 250 microliters of DMSO, extract in an ultrasonic cleaner for 30 minutes, let stand at room temperature for 24 hours, and dilute to 50 milliliters with E3 culture. Centrifuge at 4000rpm, take the supernatant, the concentration of the supernatant is 5mg / ml. Dilute with E3 culture medium to obtain a series of drug solutions with concentrations of 0.08, 0.16, 0.31, 0.63, 1.25, 2.5 and 5 mg / ml.
[0012] 2. Place zebrafish embryos 3 hours after fertilization in a 96-well plate, place 1 embryo in each well, add 200 microliters of medicinal solution to each well, and culture 30 embryos with each concentration of medicinal solution. The orifice plate is placed in an environment of 28.5 degrees Celsius and a light / dark cycle of 12 / 12 (hours) for cultivation, and the medicinal solution is renewed every 24 hours until 72 hours after fertilization.
[0013] 3. Observe the morphology of zebrafish embryos at each ...
Embodiment 2
[0015] 1. Take a few Fuyanjing capsules, remove the capsules and take the drug core powder, accurately weigh 400 mg, add 250 microliters of DMSO, extract in an ultrasonic cleaner for 30 minutes, let it stand at room temperature for 24 hours, and culture and dilute with E3 to 50 ml, centrifuge at 4000 rpm for 20 minutes, and take the supernatant, the concentration of the supernatant is 8 mg / ml. The solution was diluted with E3 culture medium to obtain drug solutions with concentrations of 0.5, 1, 2, 4, 6 and 8 mg / ml.
[0016] 2. Place zebrafish embryos 3 hours after fertilization in a 96-well plate, place 1 embryo in each well, add 200 microliters of medicinal solution to each well, and culture 30 embryos with each concentration of medicinal solution. The orifice plate is placed in an environment of 28.5 degrees Celsius and a light / dark cycle of 12 / 12 (hours) for cultivation, and the medicinal solution is updated every 24 hours until 72 hours after fertilization.
[0017] 3. O...
Embodiment 3
[0019] 1. Cut Angong Niuhuang Pills into granules, accurately weigh 250 mg, add 250 microliters of DMSO, extract in an ultrasonic cleaner for 30 minutes, let stand at room temperature for 24 hours, and dilute to 50 milliliters with E3 culture. Centrifuge at 4000rpm, take the supernatant, the concentration of the supernatant is 5mg / ml. Dilute with E3 broth to give concentrations of 0.25, 0.38, 0.5, 0.75, 1, 1.5 and 2 mg / ml.
[0020] 2. Place zebrafish embryos 3 hours after fertilization in a 96-well plate, place 1 embryo in each well, add 200 microliters of medicinal solution to each well, and culture 30 embryos with each concentration of medicinal solution. The orifice plate is placed in an environment of 28.5 degrees Celsius and a light / dark cycle of 12 / 12 (hours) for cultivation, and the medicinal solution is updated every 24 hours until 72 hours after fertilization.
[0021] 3. Observe the morphology of zebrafish embryos at each concentration under a microscope and record ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com