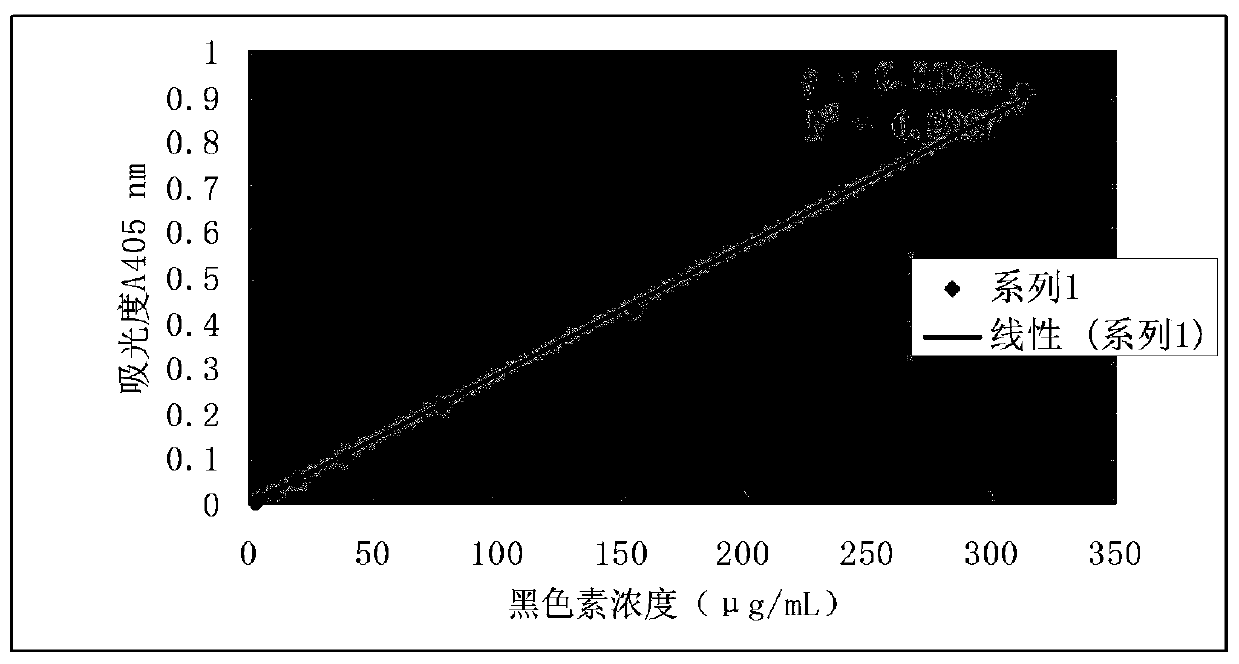
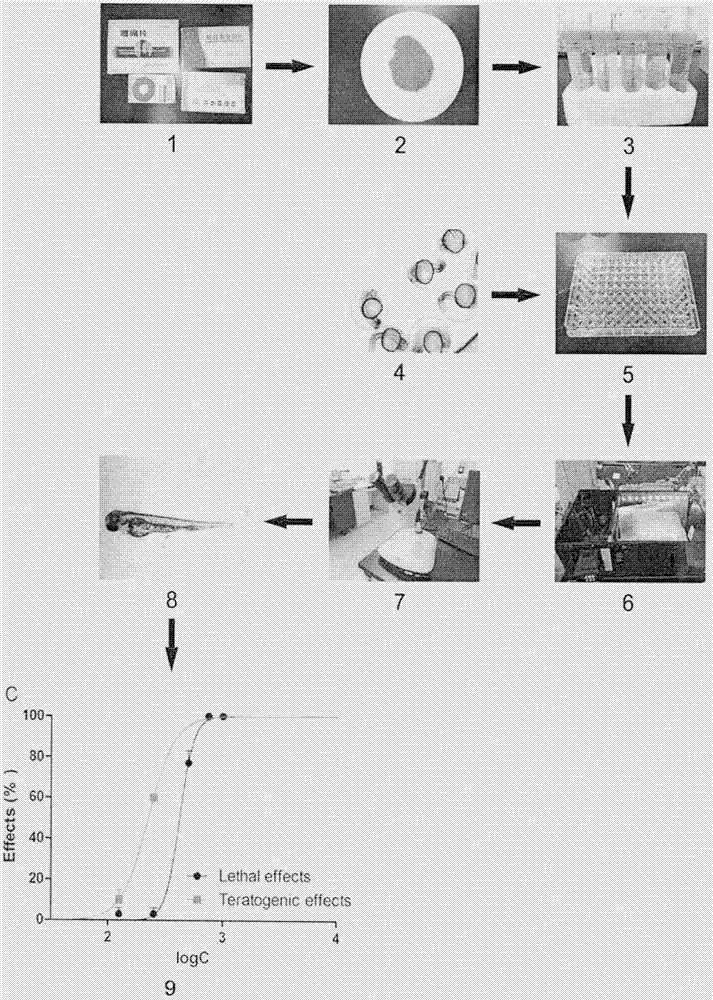
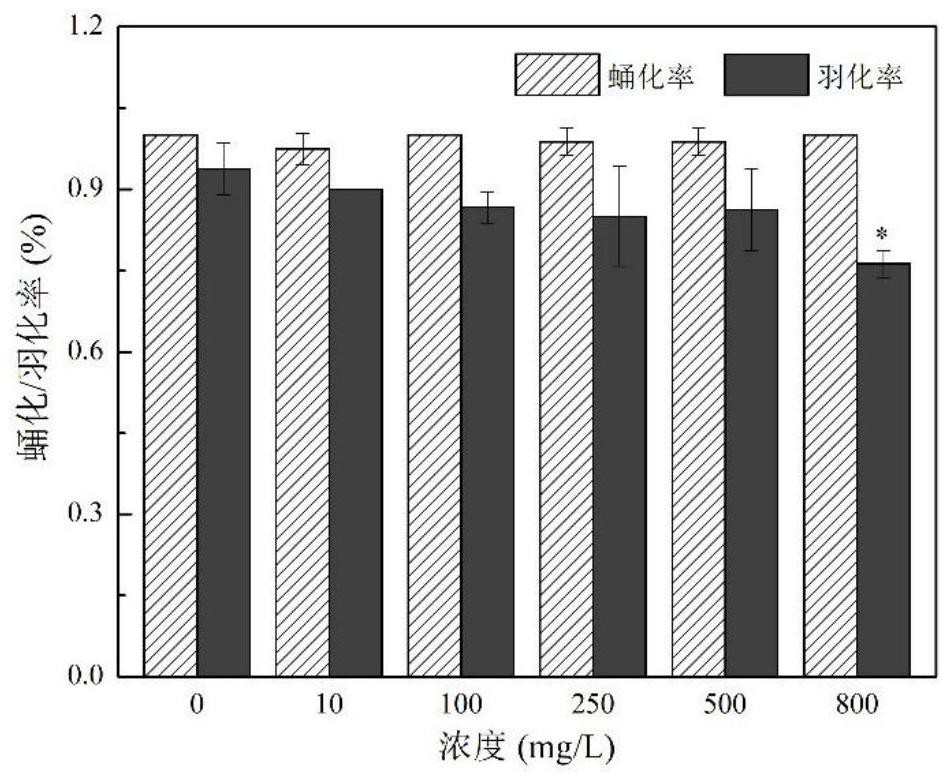
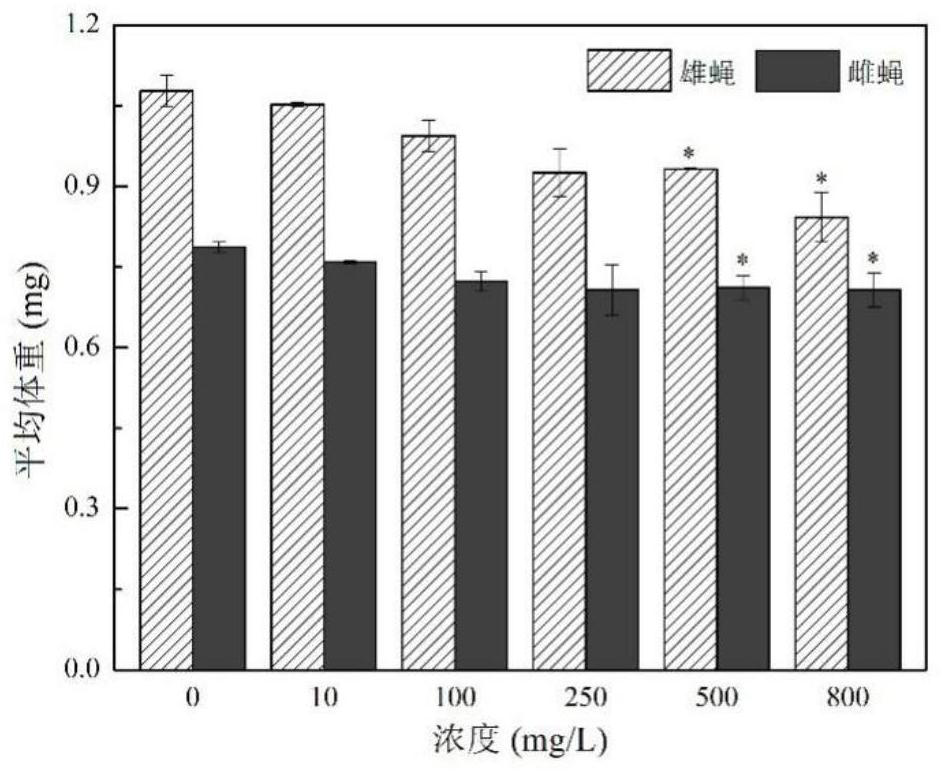
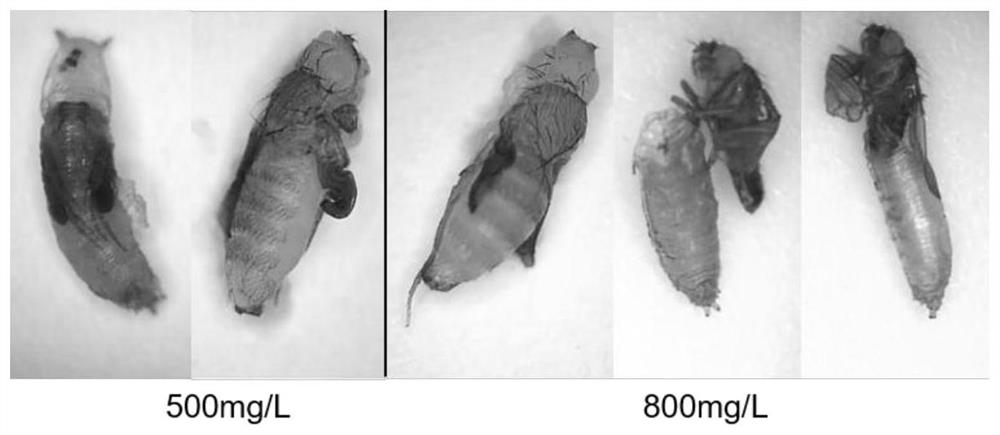
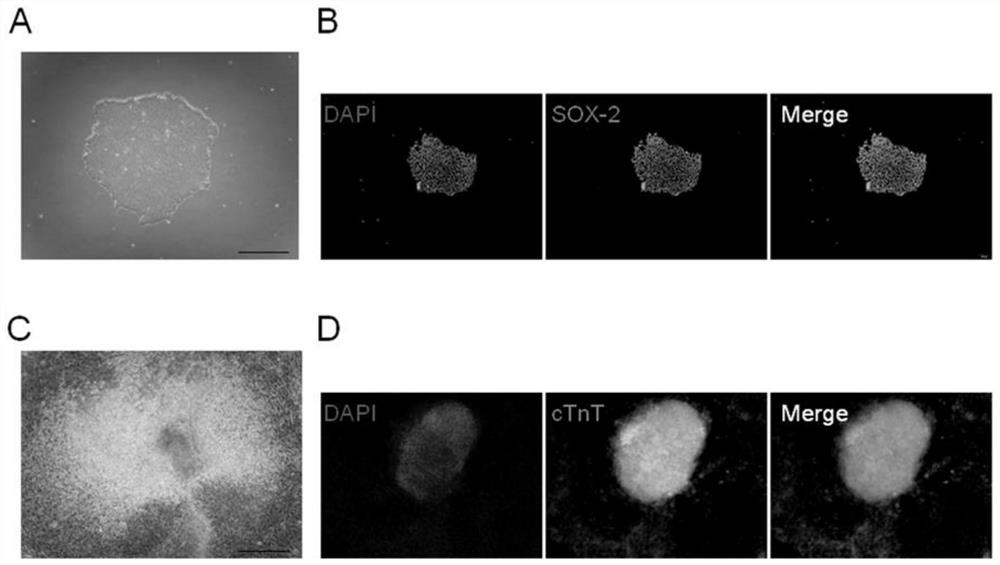
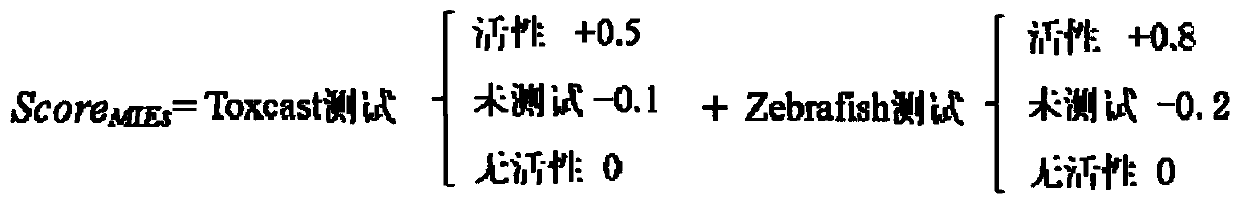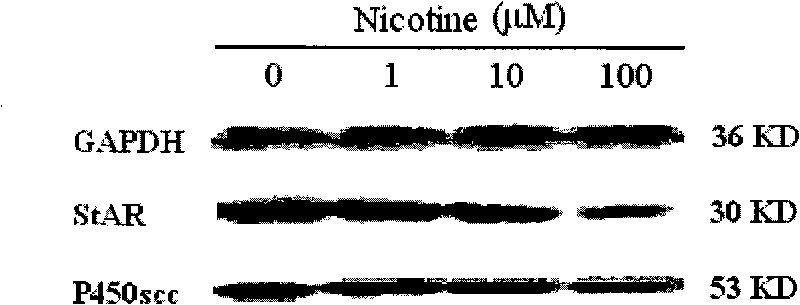Patents
Literature
31 results about "Developmental toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Developmental toxicity is any structural or functional alteration, reversible or irreversible, which interferes with homeostasis, normal growth, differentiation, development or behavior, and which is caused by environmental insult (including drugs, lifestyle factors such as alcohol, diet, and environmental toxic chemicals or physical factors). It is the study of adverse effects on the development of the organism resulting from exposure to toxic agents before conception (either parent), during prenatal development, or post-natally until puberty. The substance that causes developmental toxicity from embryonic stage to birth is called teratogens. The effect of the developmental toxicants depends on the type of substance, dose and duration and time of exposure.
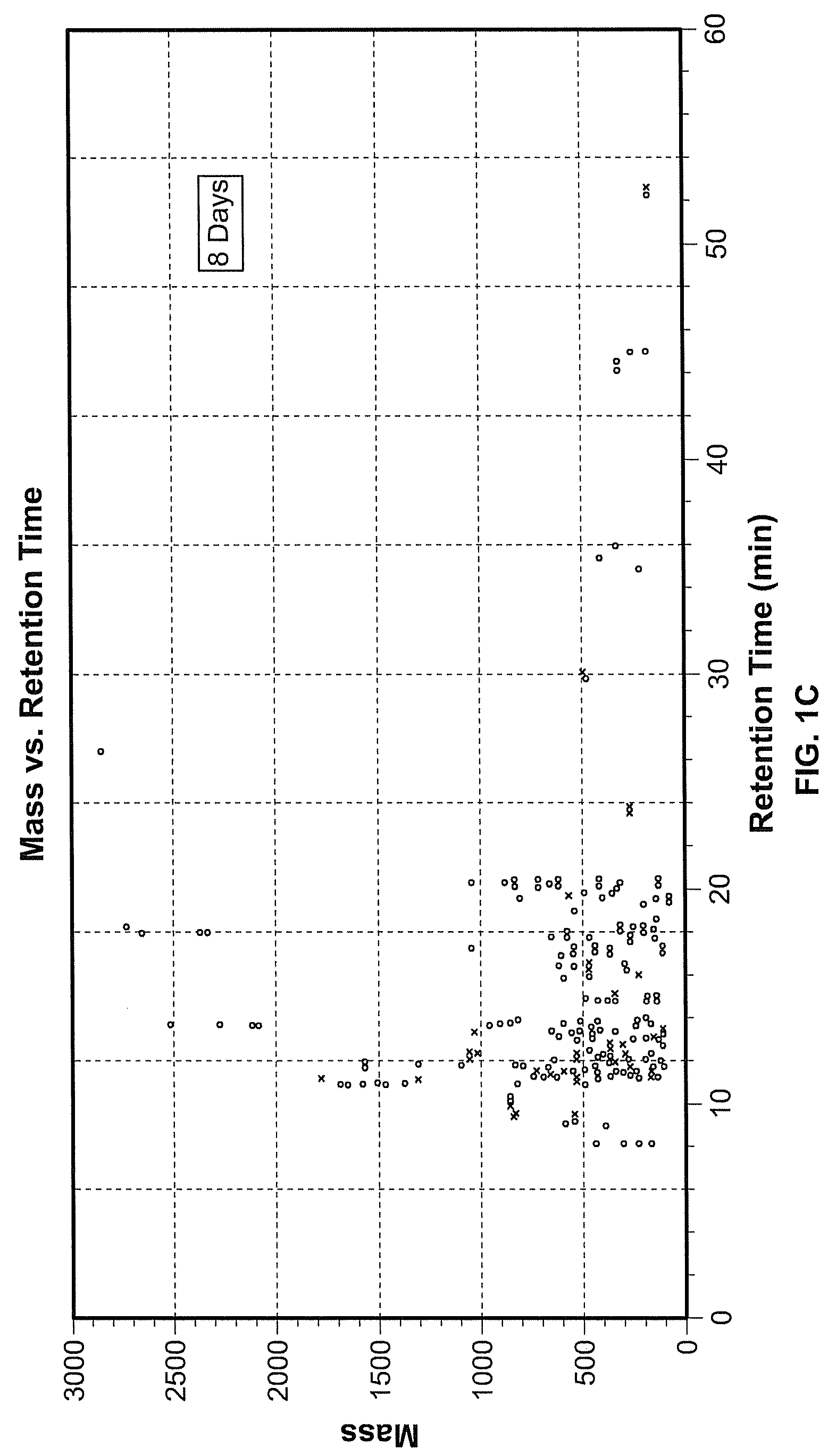
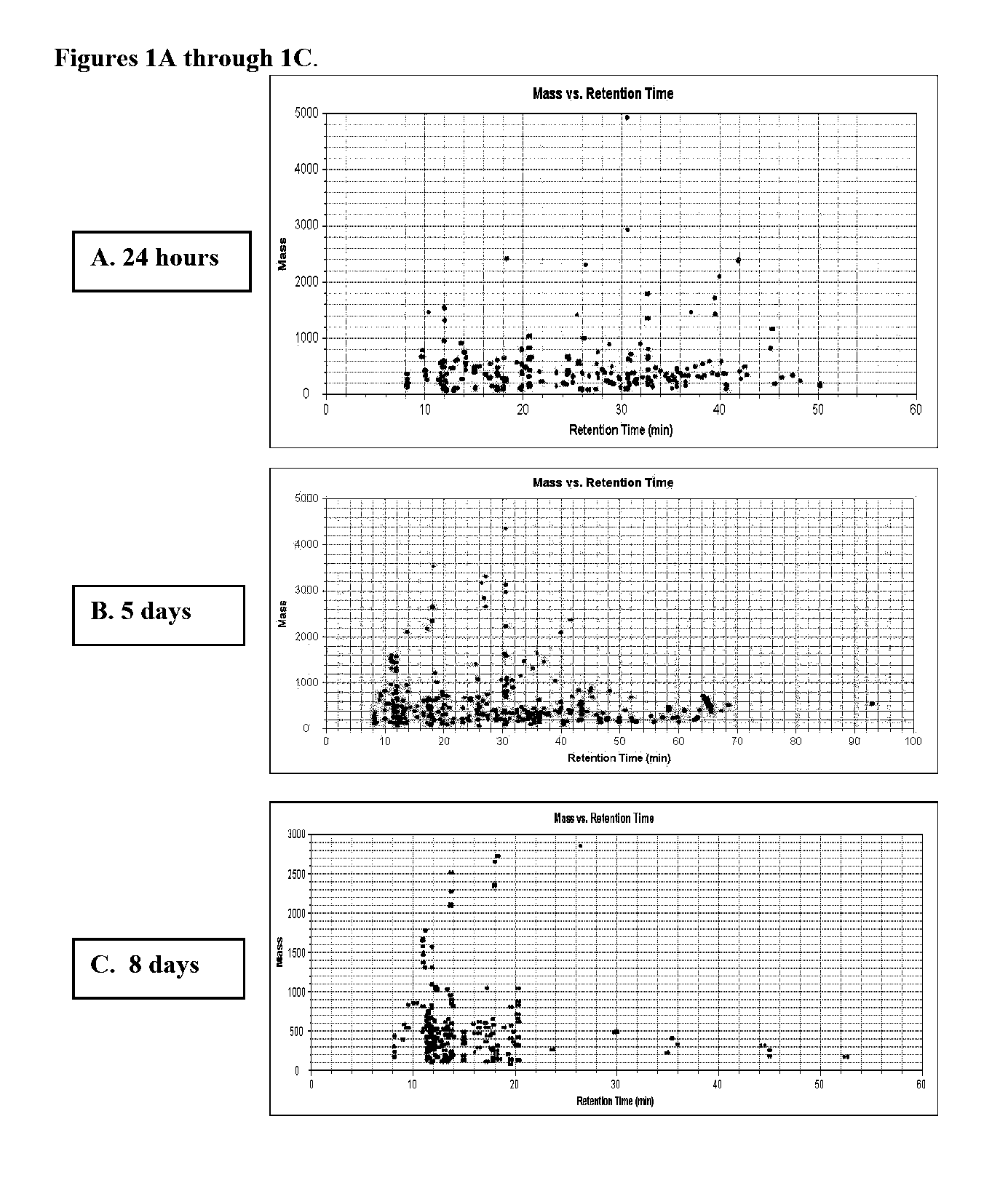
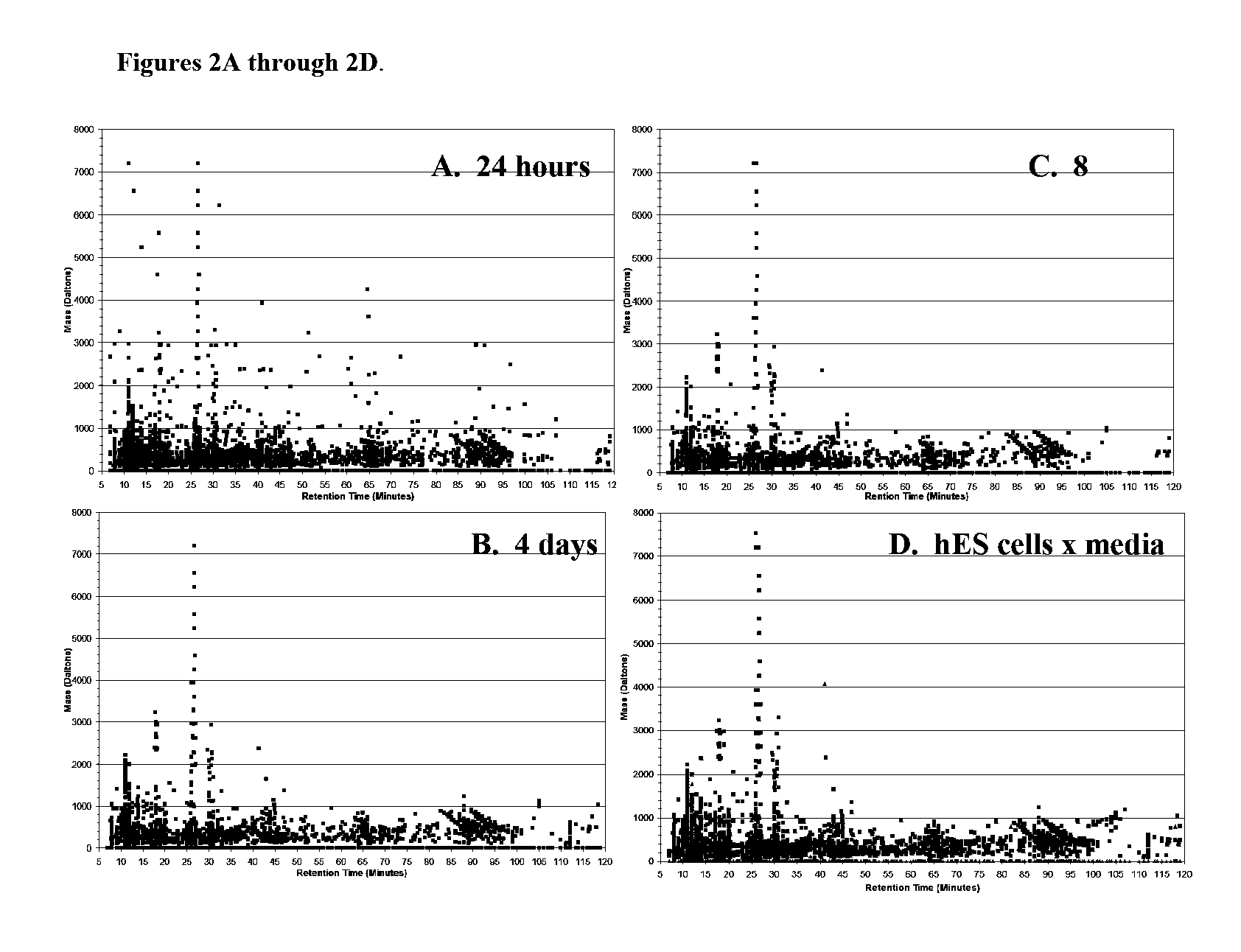
Reagents and Methods for Using Human Embryonic Stem Cells to Evaluate Toxicity of Pharmaceutical Compounds and Other Chemicals
ActiveUS20070248947A1Reliably determinedPotential for false negatives is reducedCompound screeningApoptosis detectionTesting toxicityChemical compound
The invention provides biomarker profiles of cellular metabolites and methods for screening chemical compounds including pharmaceutical agents, lead and candidate drug compounds and other chemicals using human embryonic stem cells (hESC) or lineage-specific cells produced therefrom. The inventive methods are useful for testing toxicity, particularly developmental toxicity and detecting teratogenic effects of such chemical compounds.
Owner:WISCONSIN ALUMNI RES FOUND
Predicting Human Developmental Toxicity of Pharmaceuticals Using Human Stem-Like Cells and Metabolomics
ActiveUS20110312019A1Increased toxicityMicrobiological testing/measurementMaterial analysisTesting toxicityMetabolite
The invention provides biomarker profiles of metabolites and methods for screening chemical compounds including pharmaceutical agents, lead and candidate drug compounds and other chemicals using human stem-like cells (hSLCs) or lineage-specific cells produced therefrom. The inventive methods are useful for testing toxicity, particularly developmental toxicity and detecting teratogenic effects of such chemical compounds. Specifically, a more predictive developmental toxicity model, based on an in vitro method that utilizes both hSLCs and metabolomics to discover biomarkers of developmental toxicity is disclosed.
Owner:STEMINA BIOMARKER DISCOVERY
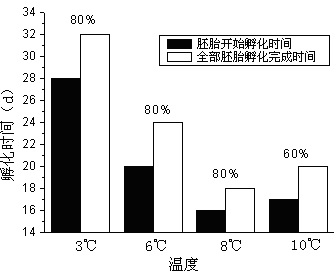
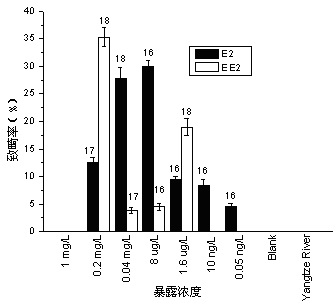
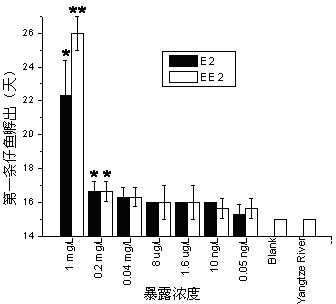
Method for testing toxicity of environmental estrogen on whitebait embryonic development
The invention discloses a method for testing toxicity of environmental estrogen on whitebait embryonic development. The method of the invention utilizes the sensitivity of embryo to the simulation of environmental pollutant or chemical matter to perform a test under semi-static state on whitebait embryo which is exposed in standard diluted aqueous solution of subject having a series of concentrations. The test lasts about 30 days, begins as exposing a live embryo at the blastula stage in the aqueous solution of subject, and ends with hatching all embryos in a control group and an exposed group. The toxicity endpoint contains death, monstrosity and delayed hatching. The method comprises the steps of confirming the toxicity of the subject on the whitebait embryonic development by observing the toxicity endpoint of the exposed group and comparing with the control group; researching on the influence of two natural estrogens E2 and EE2 on the whitebait embryo-yolk sac stage so as to confirm the toxic dose-effect relationship of the natural estrogens to the whitebait embryonic development, and evaluating the potential risk of this type of fish exposed under the estrogen with environmental concentration.
Owner:SHANGHAI ACADEMY OF ENVIRONMENTAL SCIENCES +1
Predicting human developmental toxicity of pharmaceuticals using human stem-like cells and metabolomics
ActiveUS8703424B2Increased toxicityMicrobiological testing/measurementTissue cultureTesting toxicityMetabolite
The invention provides biomarker profiles of metabolites and methods for screening chemical compounds including pharmaceutical agents, lead and candidate drug compounds and other chemicals using human stem-like cells (hSLCs) or lineage-specific cells produced therefrom. The inventive methods are useful for testing toxicity, particularly developmental toxicity and detecting teratogenic effects of such chemical compounds. Specifically, a more predictive developmental toxicity model, based on an in vitro method that utilizes both hSLCs and metabolomics to discover biomarkers of developmental toxicity is disclosed.
Owner:STEMINA BIOMARKER DISCOVERY
Method for detecting development toxicity of environmental pollutants by whole-mount in-situ hybridization technique
InactiveCN106282359AShort detection cycleTo achieve the purpose of early warningMicrobiological testing/measurementFish embryoEmbryo
The invention belongs to the technical field of biology, and particularly relates to a method for detecting development toxicity of environmental pollutants by a whole-mount in-situ hybridization technique. The method comprises the following step: detecting the development toxicity of environmental pollutants to zebra fish embryos by a whole-mount in-situ hybridization technique. The method can be used for predicting potential toxic actions of the environmental pollutants on zebra fish organs and tissues, thereby further disclosing the action mode of the environmental pollutants, shortening the detection period, and achieving the goal of early warning in deed.
Owner:CHONGQING INST OF GREEN & INTELLIGENT TECH CHINESE ACADEMY OF SCI
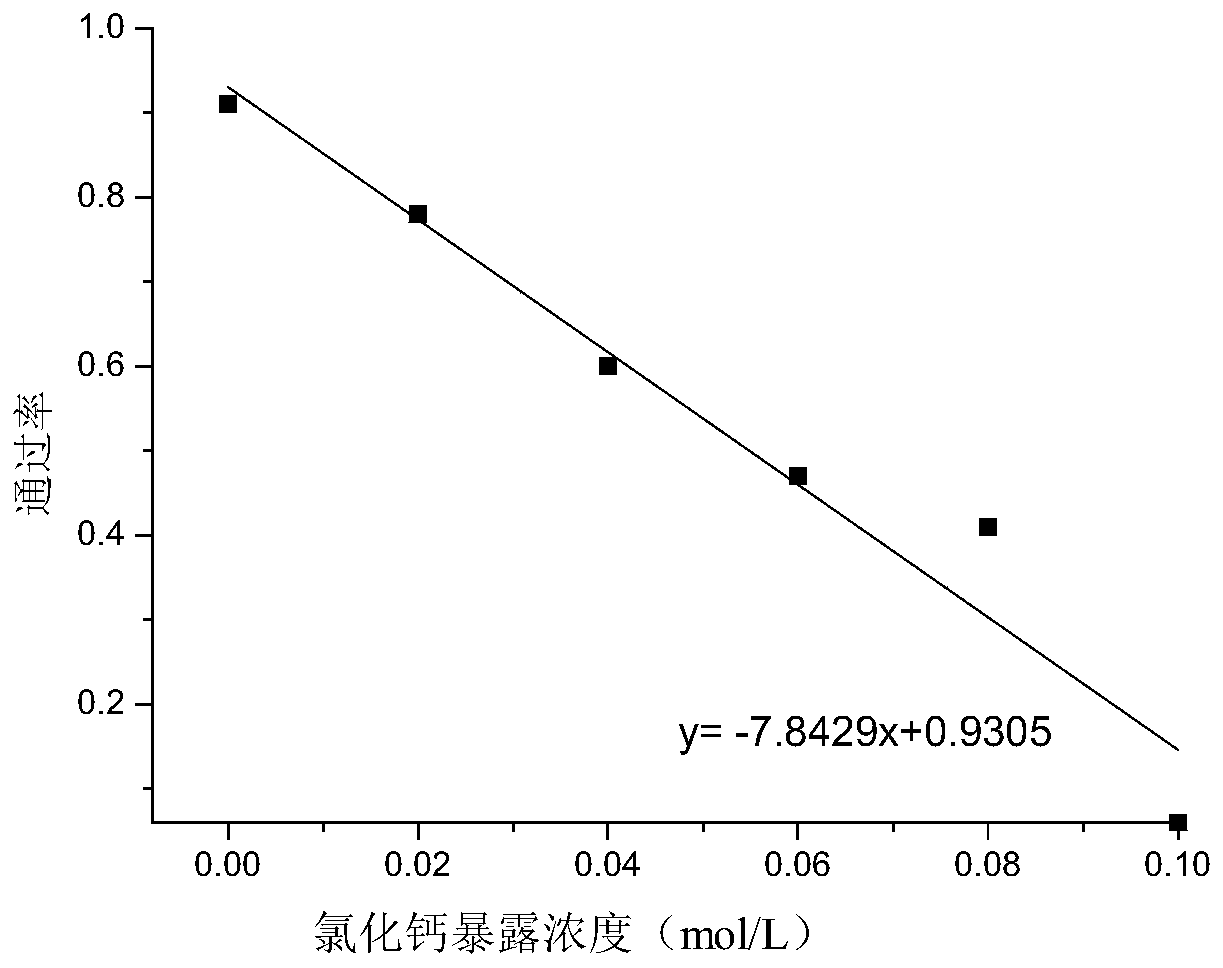
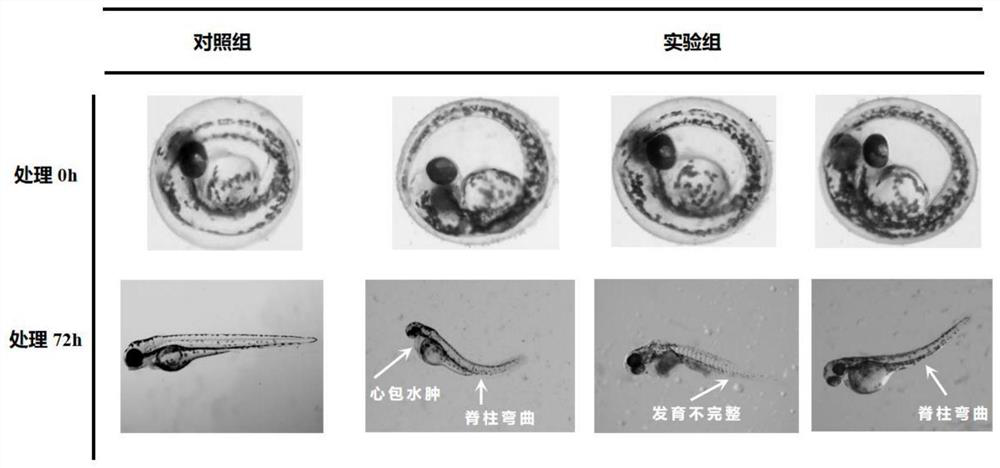
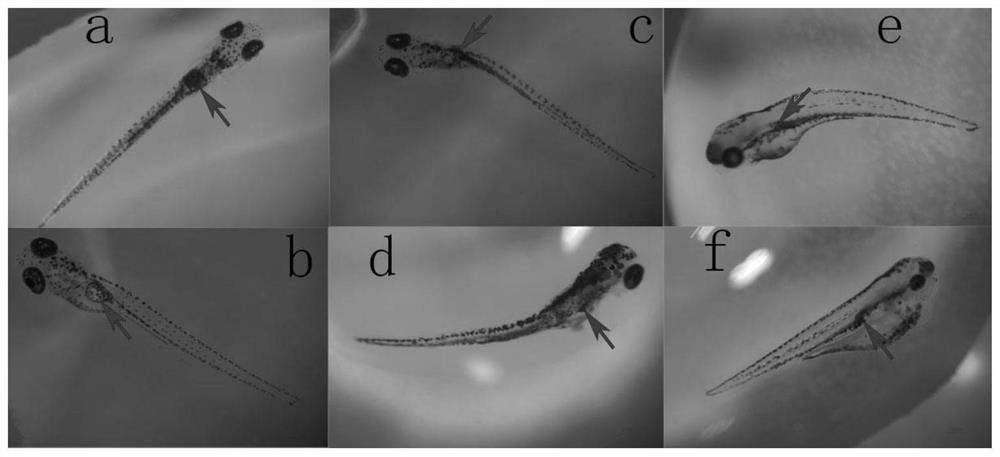
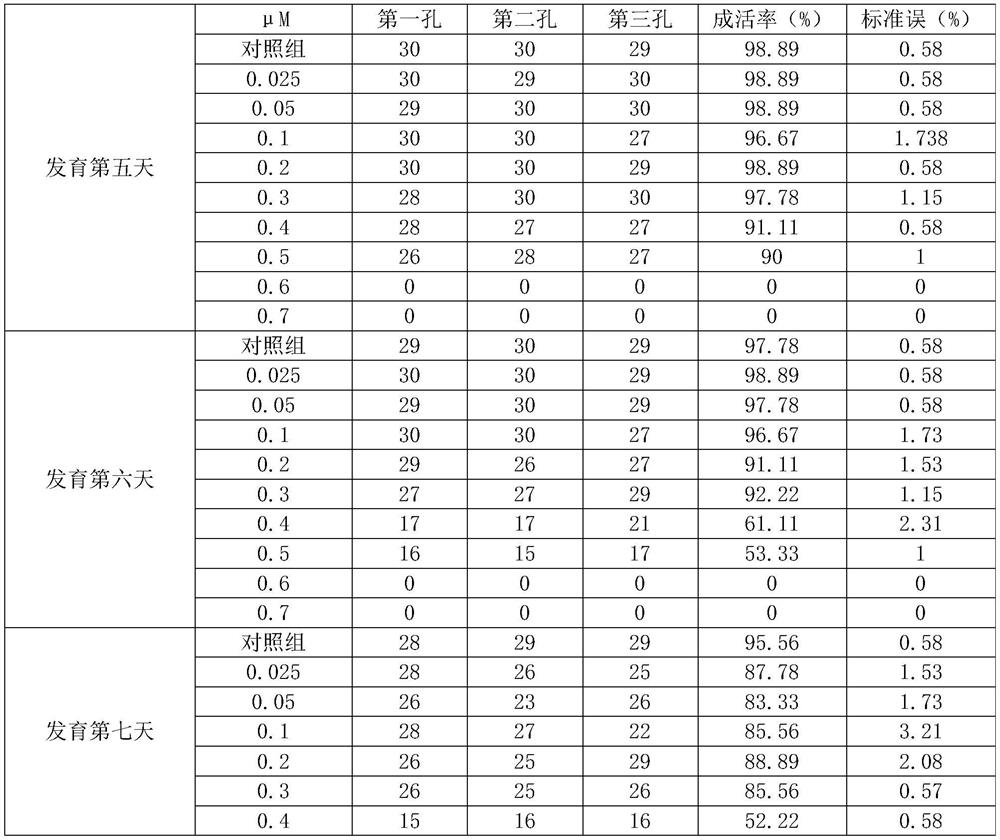
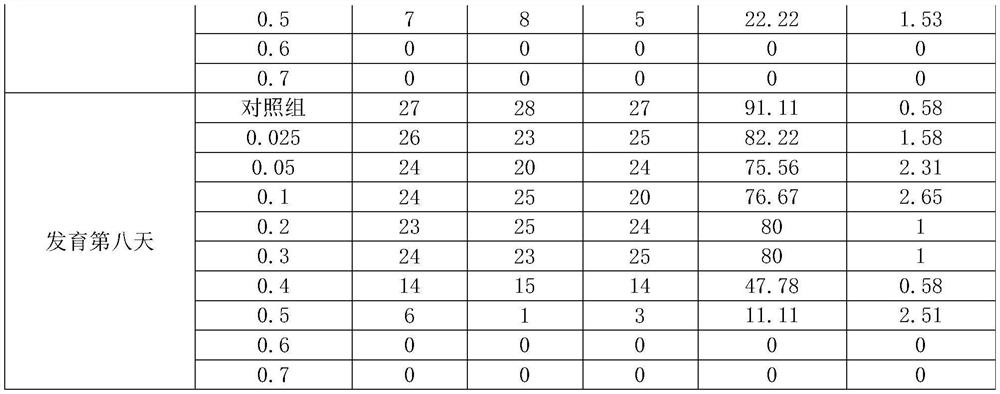
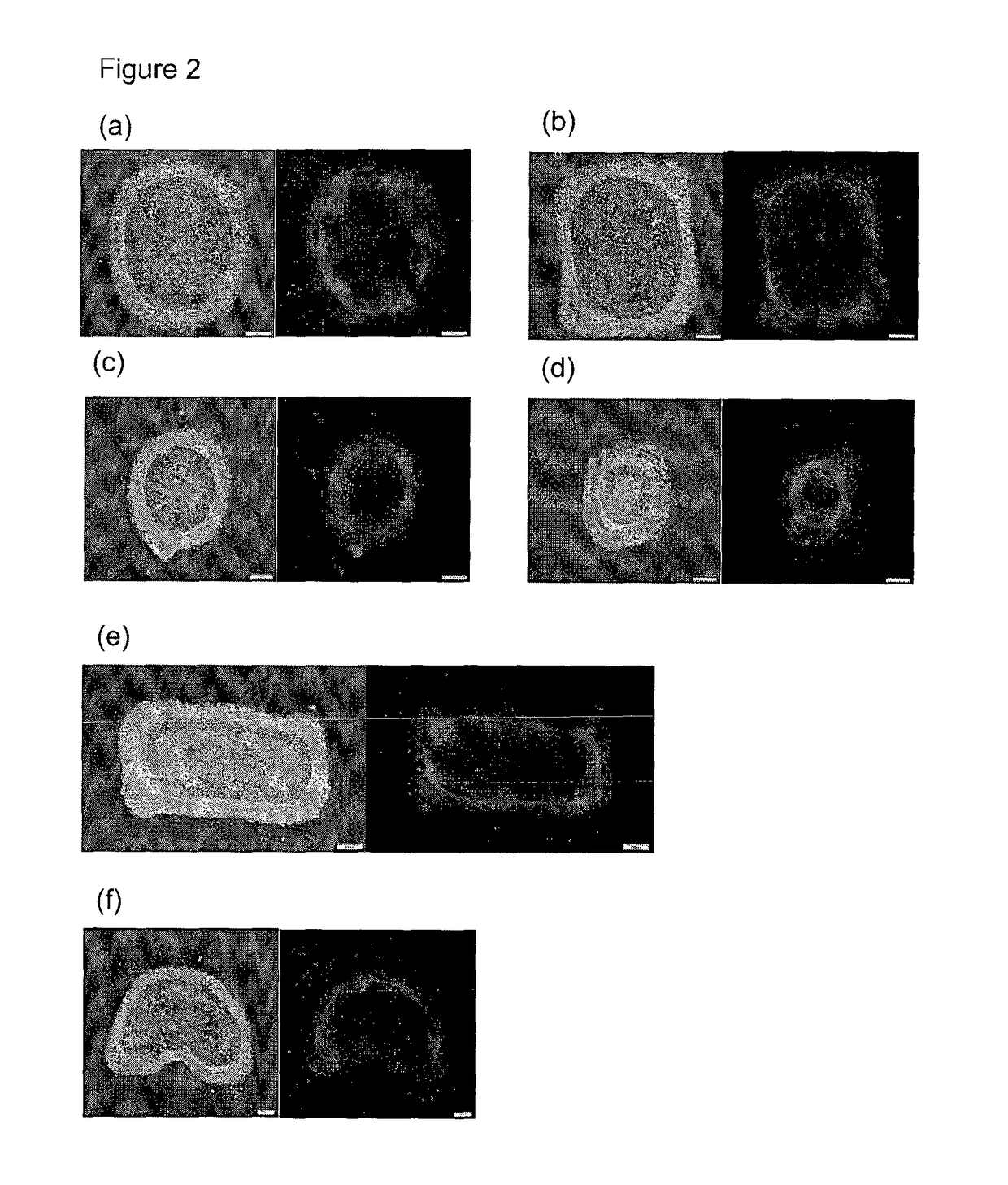
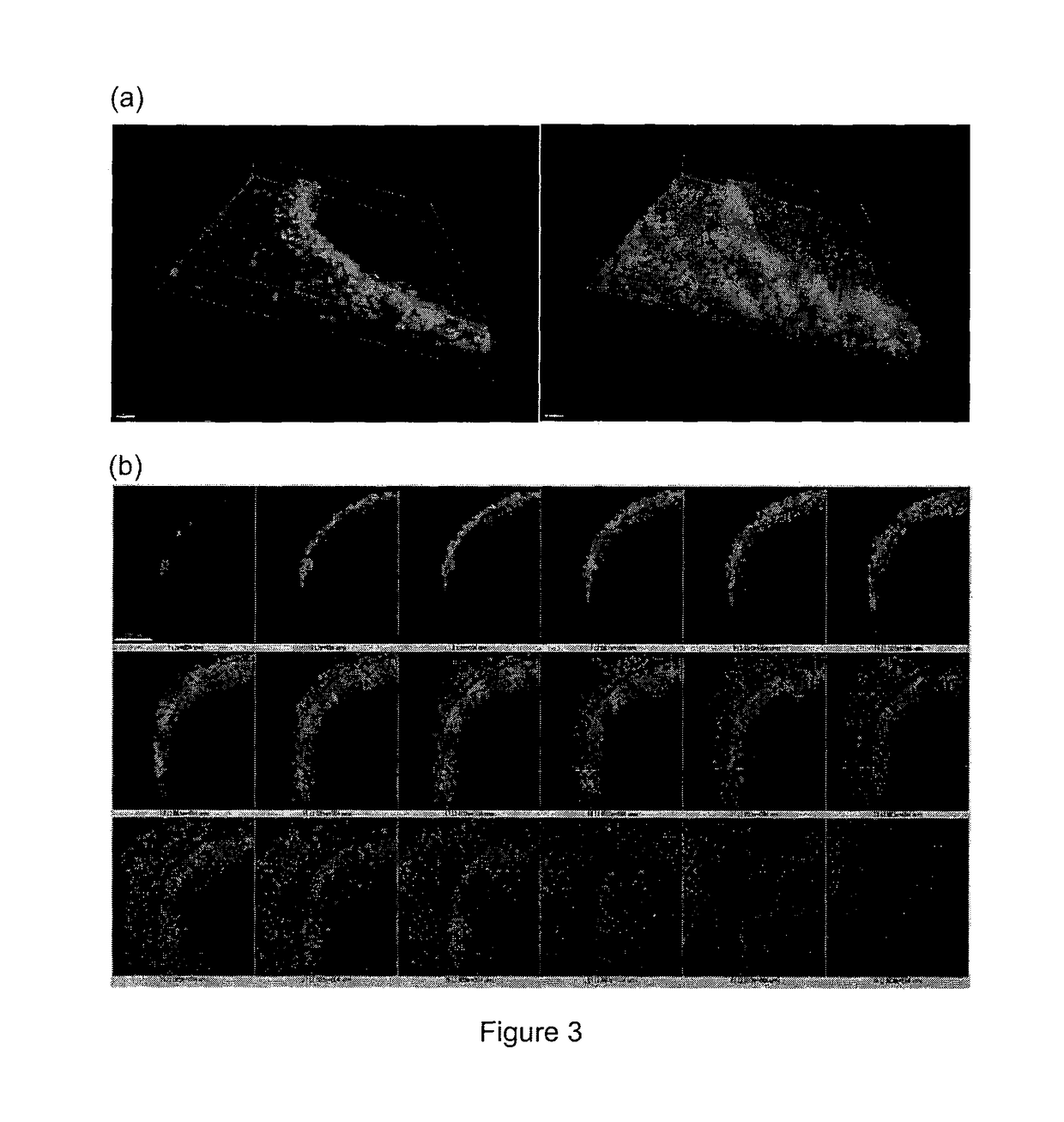
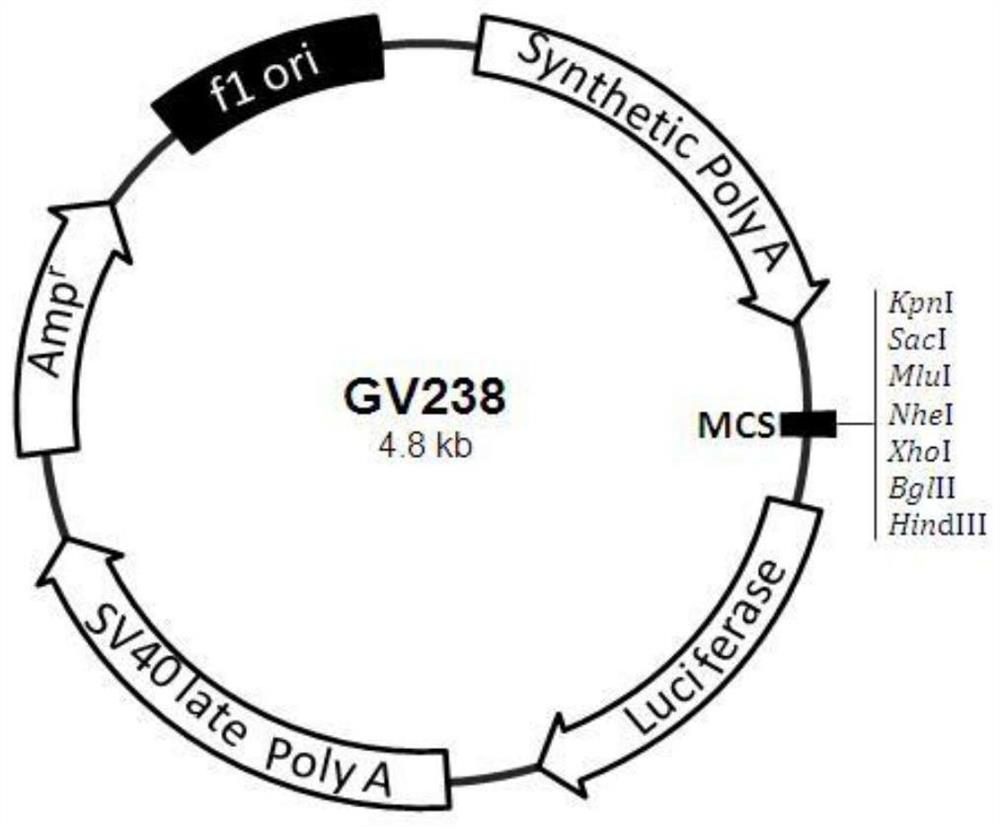
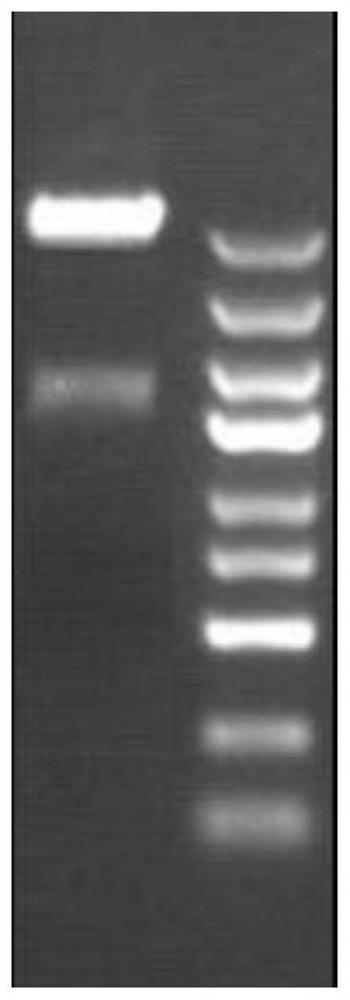
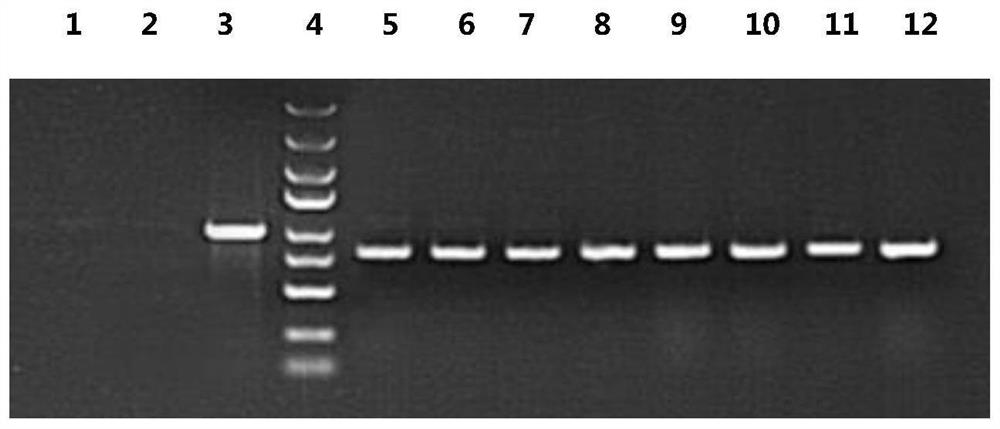
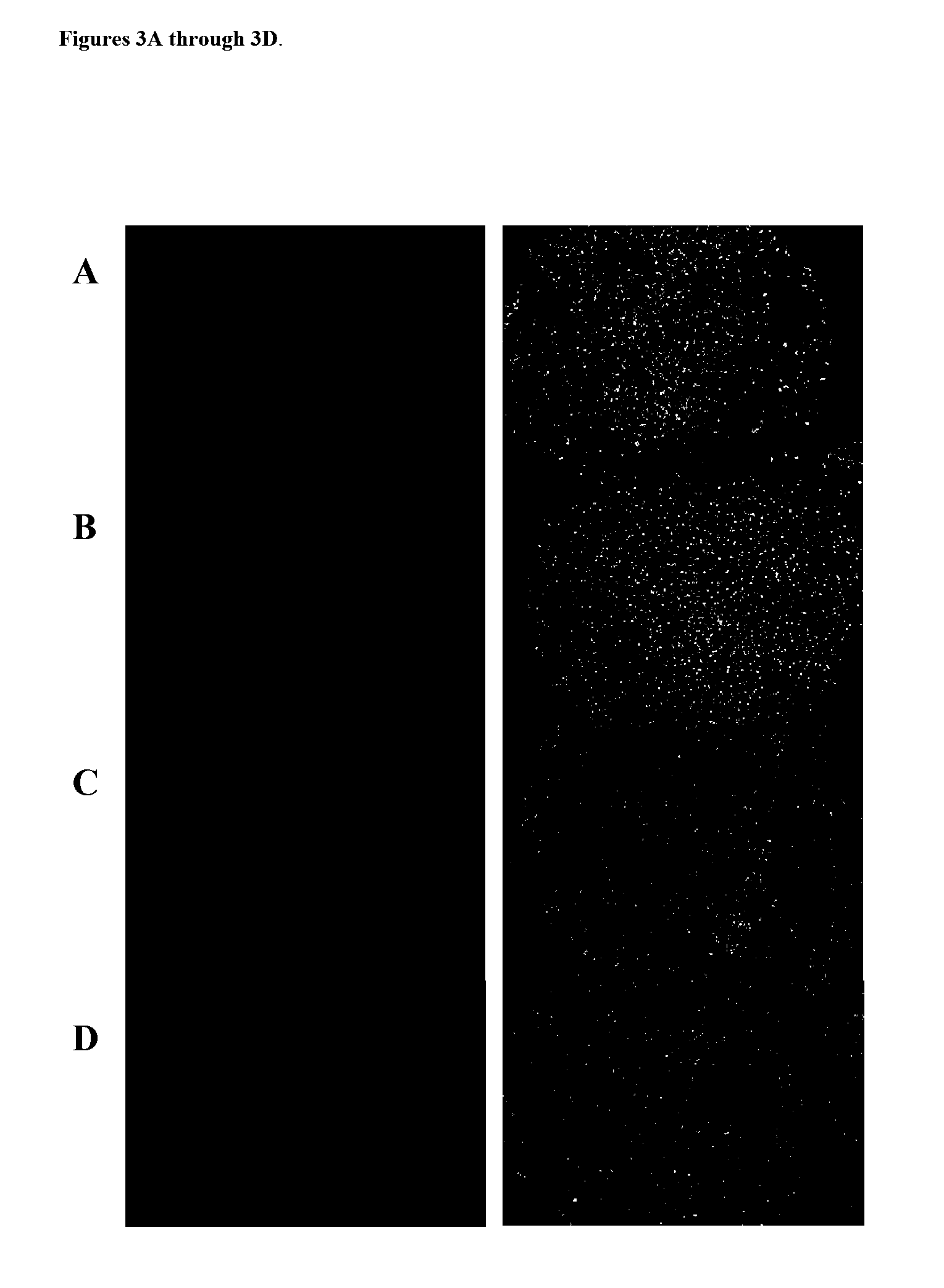
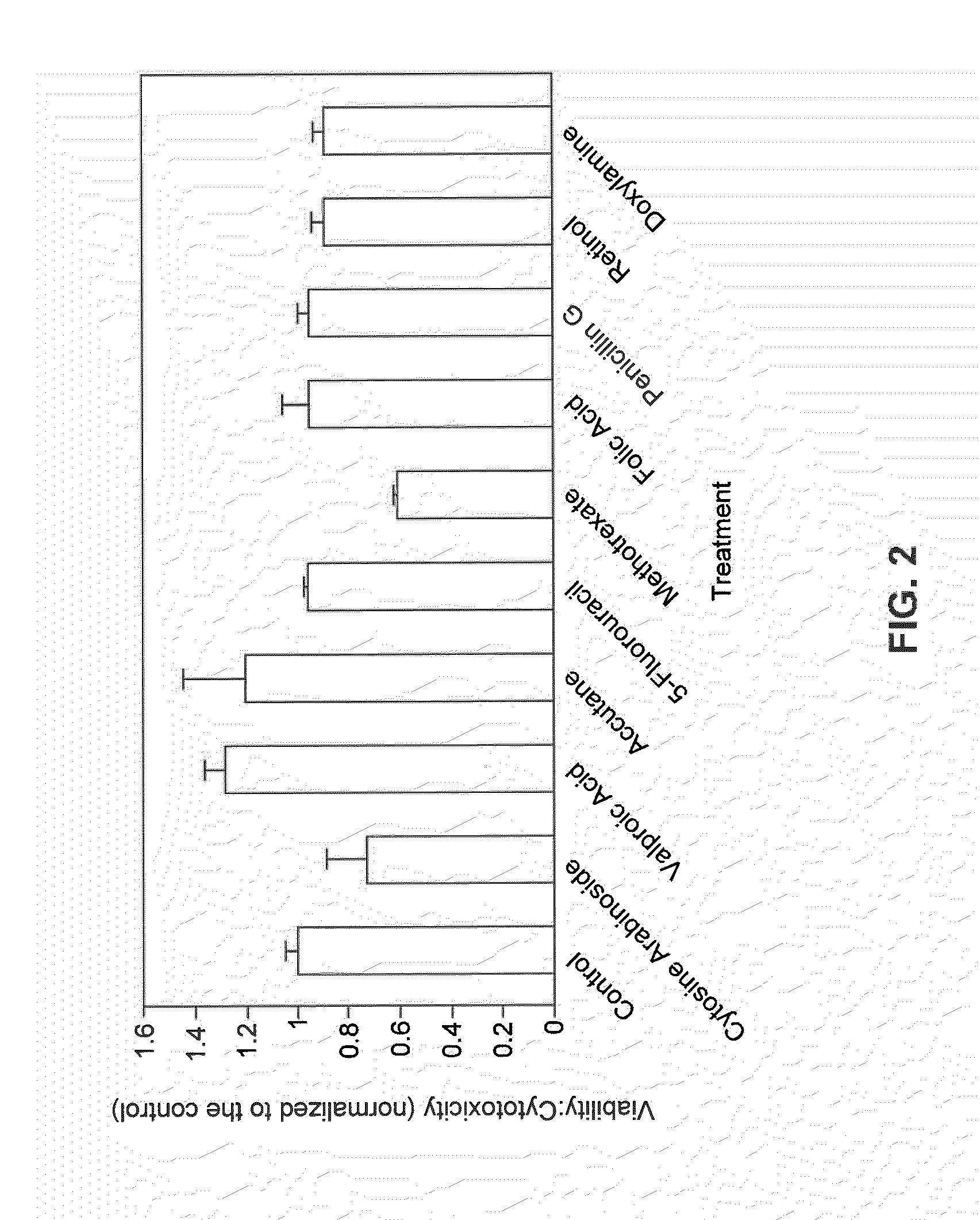
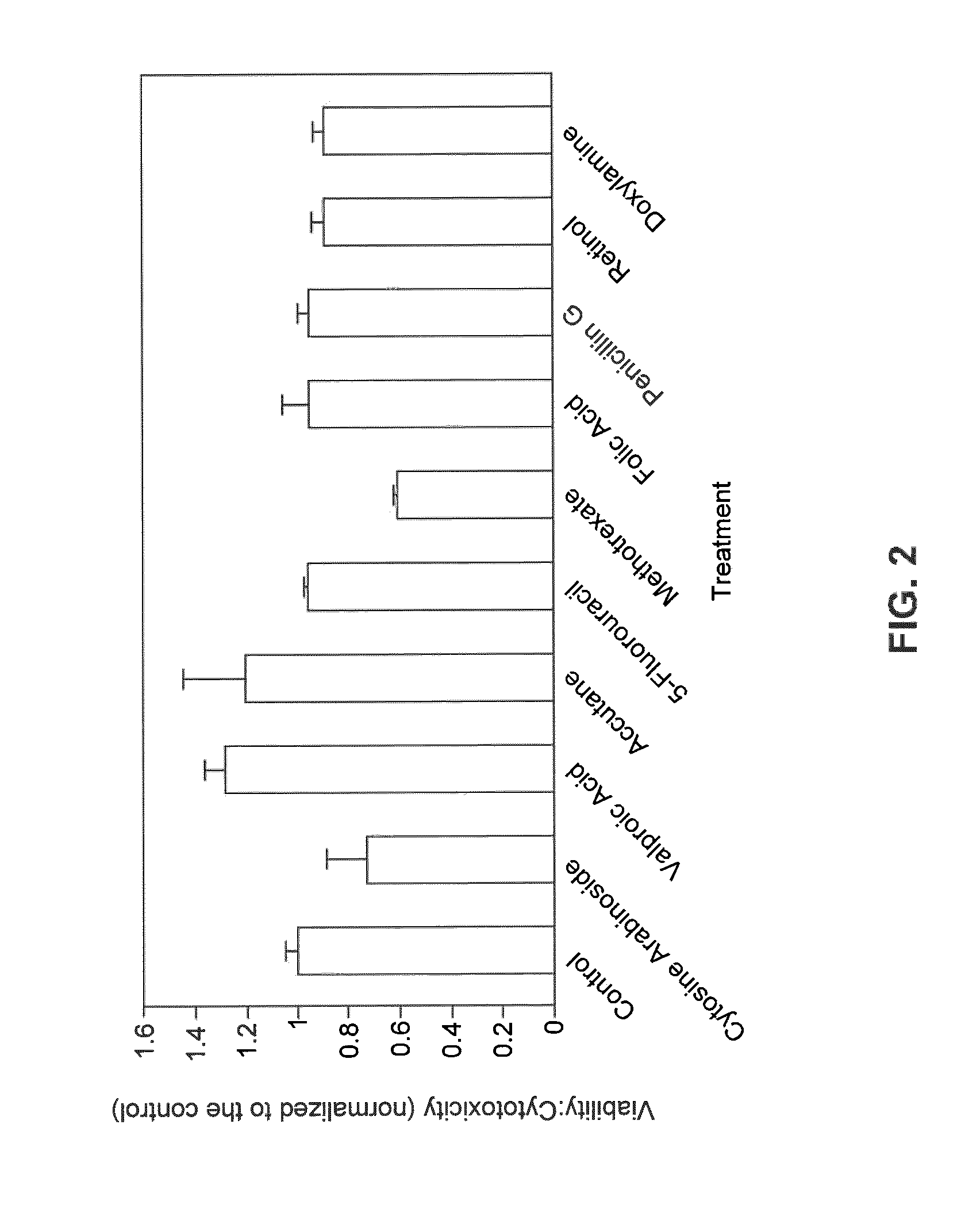
Kit for screening drug developmental toxicity based on C/EBP[alpha] and IGF1R gene and application thereof
ActiveCN107841556AReliable and reliableAdaptableMicrobiological testing/measurementCell lysatesMrna expression
The invention relates to a kit for screening drug developmental toxicity based on a C / EBP[alpha]-IGF1R gene and an application thereof. The kit contains: primers for detecting expression of C / EBP[alpha] and IGF1R gene mRNA; drug acting object mammalian cell lines or differentiated cells, and cell lines or differentiated cells having a plasmid containing an IGF1R promoter and a luciferase reportergene transferred into cells; a cell lysate; and luciferase. The determination standard of the kit comprises improvement of intracellular C / EBP[alpha] gene expression of a to-be-screened compound, reduction of IGF1R gene expression, and reduction of the activity of the luciferase reporter gene in the cells containing the IGF1R promoter-reporter gene, and the to-be-screened compound is suggested tohave developmental toxicity. The kit is novel, efficient and reliable, and can be used for high-throughput drug screening, and is of great significance for rapid detection of drugs and other compoundswith developmental toxicity.
Owner:WUHAN UNIV
Method for evaluating developmental toxicity of high-concentration glucose by using zebra fish
PendingCN113736844AShort cycleImprove accuracyMicrobiological testing/measurementClimate change adaptationBiotechnologyHigh concentration
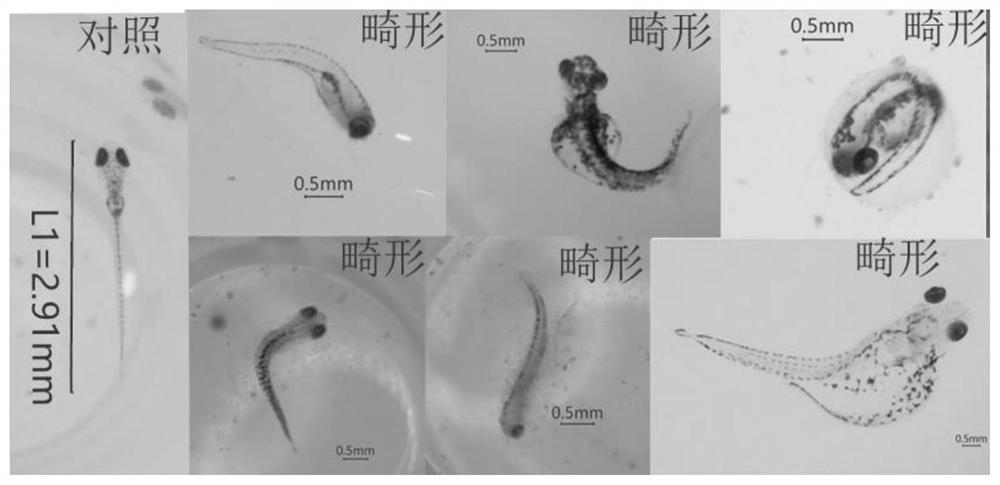
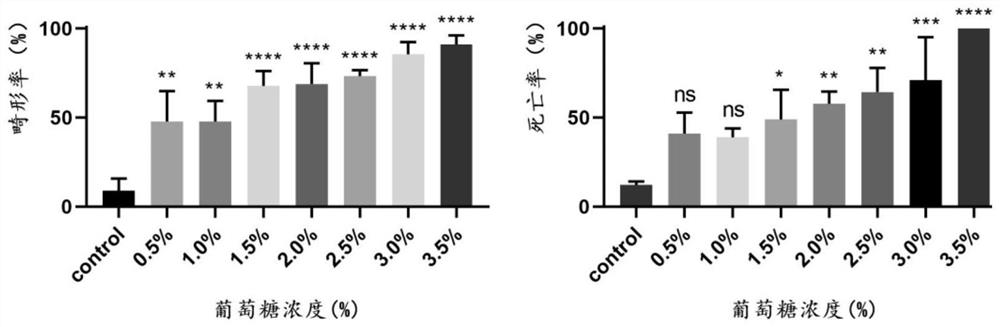
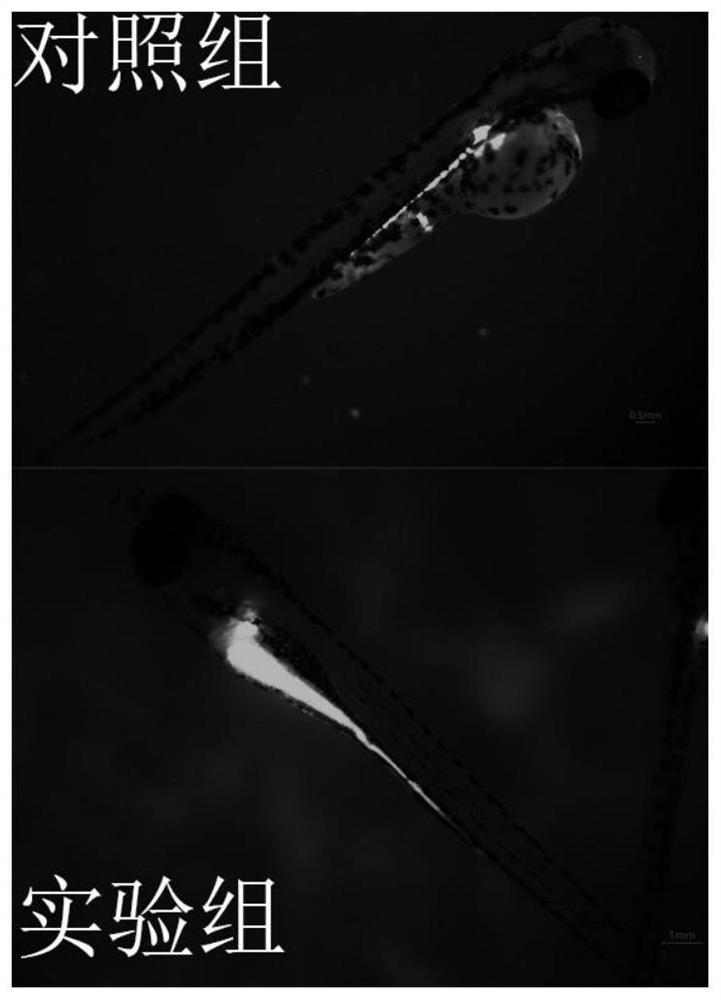
The invention discloses a method for evaluating developmental toxicity of high-concentration glucose by using zebra fish, which comprises the following steps: culturing zebra fish fertilized eggs in glucose culture solutions with different concentrations, counting the spine deformity rate and death rate of juvenile zebra fish by using glucose with different concentrations, observing the growth form of the zebra fish, and investigating the developmental toxicity of the high-concentration glucose in combination with the active oxygen generation amount, the cell apoptosis degree and the like of the juvenile fish. The method provided by the invention is simple and convenient to operate, short in evaluation period and capable of efficiently and intuitively evaluating the developmental toxicity of the high-concentration glucose by using the zebra fish, not only can evaluate the developmental toxicity of the high-concentration glucose on the zebra fish, but also can provide reference for the pathological mechanism research of the developmental toxicity of the high-concentration glucose on other organisms, especially human.
Owner:WENZHOU UNIVERSITY
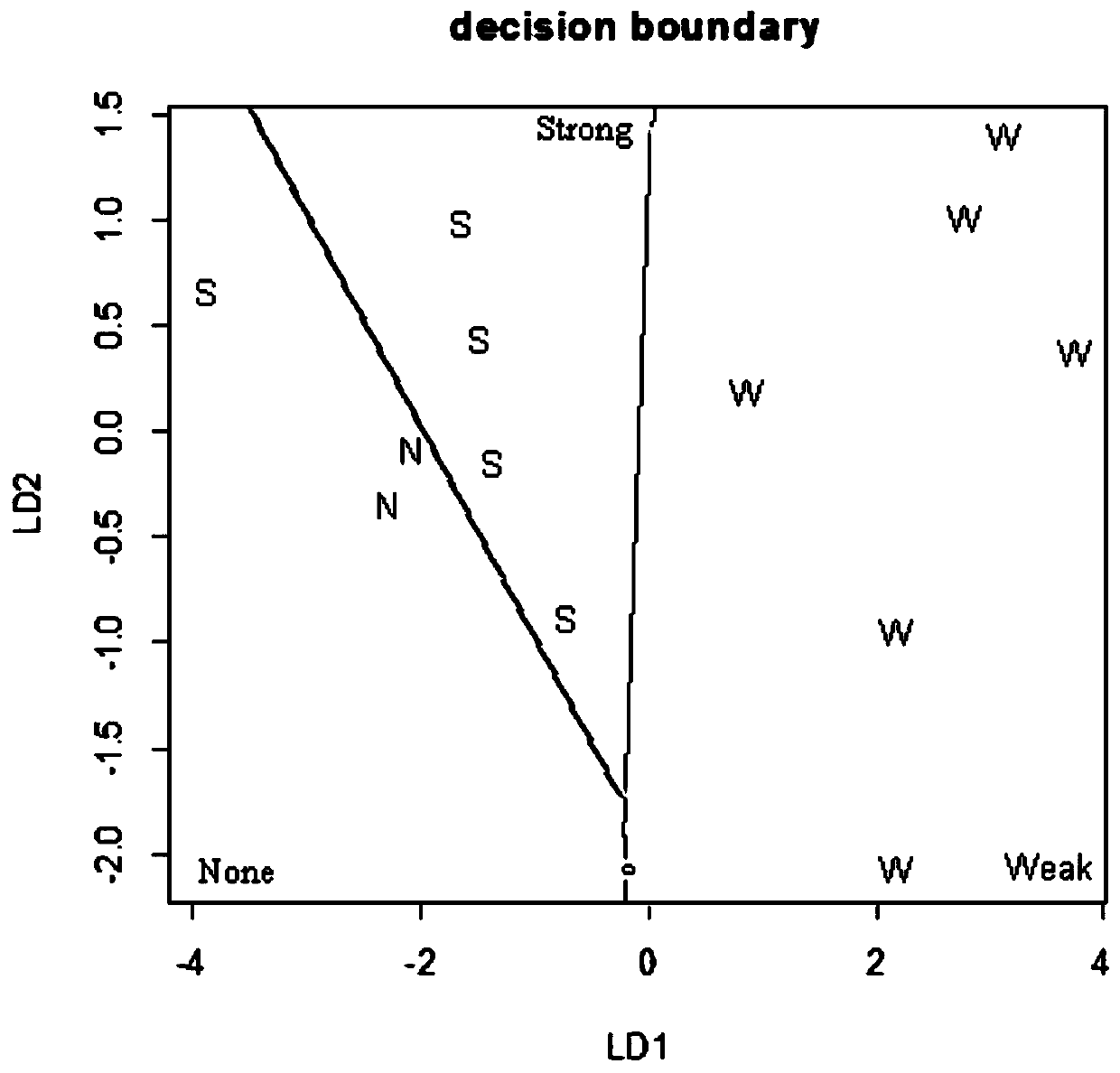
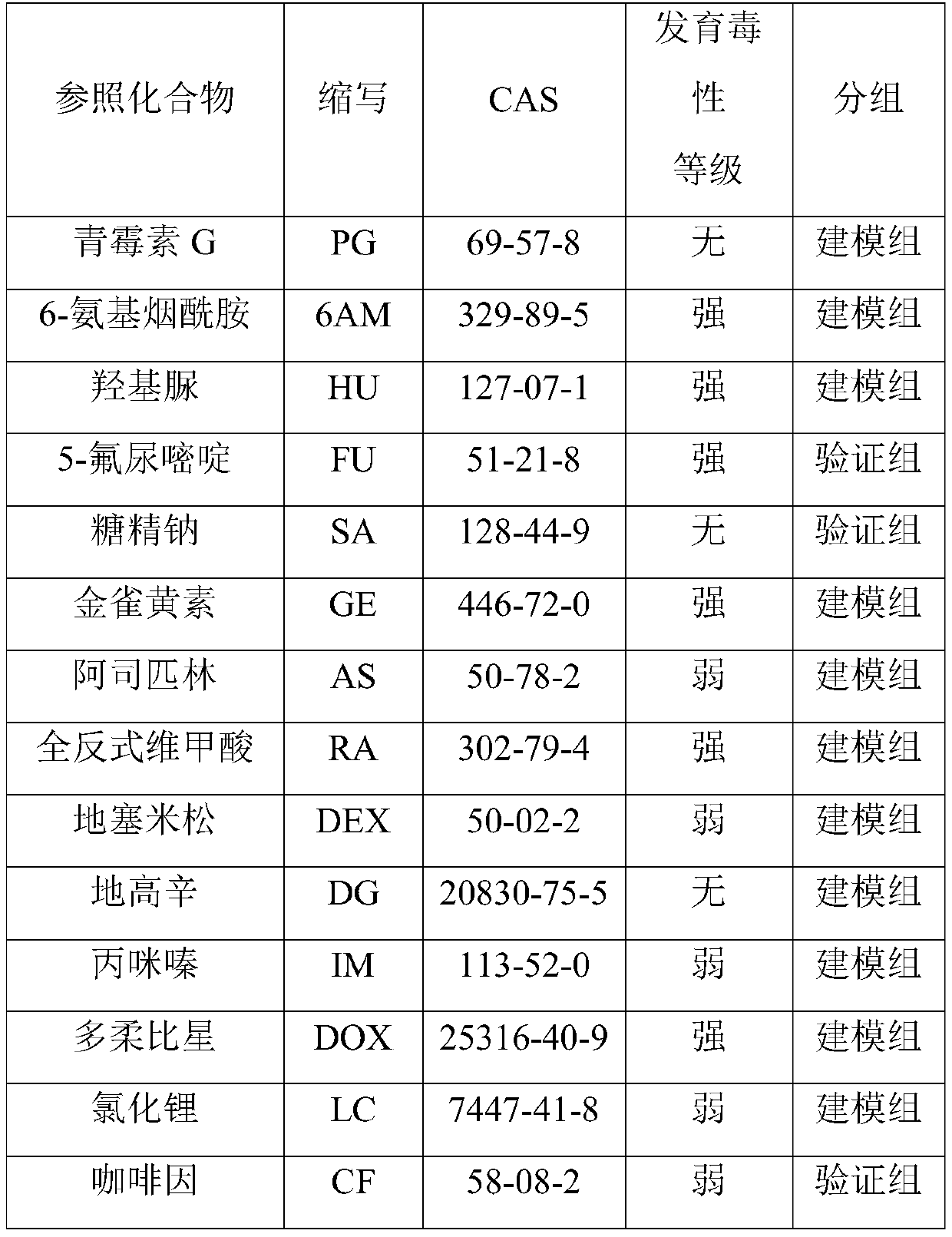
Method for detecting developmental toxicity of chemicals
ActiveCN110208516AVerify validityVerify reliabilityBiological testingHeart muscle extractIn vitro test
The invention provides a method for detecting developmental toxicity of chemicals. The method at least comprises the following steps: inducing the undifferentiated human embryonic stem cells to differentiate into myocardial cells under the culture conditions of a chemical to be detected and no chemical to be detected respectively; respectively measuring the expression quantity of MYL4 in the myocardial cells under the culture conditions of a chemical to be detected and no chemical to be detected; obtaining the expression quantity change of MYL4 in the myocardial cells under the culture condition of the chemical to be detected, namely ID, compared with the culture condition of no chemical to be detected; and according to the value of the ID, combining a Fisher discriminant function equationto judge the developmental toxicity of the chemical to be detected. The method for detecting developmental toxicity of chemicals utilizes human embryonic stem cells to establish a developmental toxicity test model for the first time, and can obtain developmental toxicity information of a compound through in vitro tests in a short time; and the method for detecting developmental toxicity of chemicals can quickly detect and prompt whether a certain chemical has developmental toxicity information for human or not.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Reagents and methods for using human embryonic stem cells to evaluate toxicity of pharmaceutical compounds and other chemicals
ActiveUS8703483B2Reliably determinedPotential for false negatives is reducedCompound screeningApoptosis detectionTesting toxicityDrug compound
The invention provides biomarker profiles of cellular metabolites and methods for screening chemical compounds including pharmaceutical agents, lead and candidate drug compounds and other chemicals using human embryonic stem cells (hESC) or lineage-specific cells produced therefrom. The inventive methods are useful for testing toxicity, particularly developmental toxicity and detecting teratogenic effects of such chemical compounds.
Owner:WISCONSIN ALUMNI RES FOUND
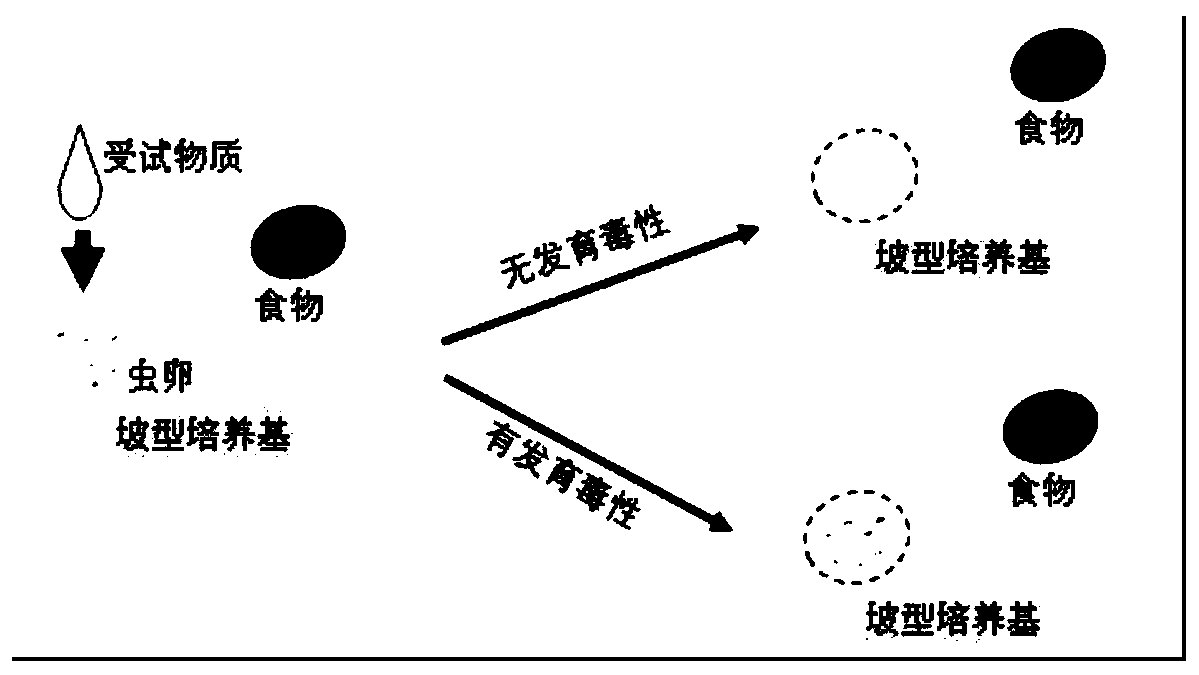
Method for detecting developmental toxicity by using caenorhabditis elegans
PendingCN110863030AQualitative judgment of developmental toxicityShorten detection timeMicrobiological testing/measurementMaterial analysisBiotechnologyEscherichia coli
The invention provides a method for detecting developmental toxicity by using caenorhabditis elegans, which belongs to the field of ecological risk evaluation, according to the invention, the method comprises the following steps: using a 24-hole cell culture plate as a main exposure environment, preparing an NGM culture medium with a specific gradient, exposing a test substance to the caenorhabditis elegans eggs on one side of the culture medium, using the sensitivity of caenorhabditis elegans to the external environment change in the development stage from eggs to adults, using escherichia coli food for inducing nematodes to generate movement behaviors, detecting the developmental toxicity according to the characteristics of development and movement, realizing direct observation even without assistance of a microscope, and quickly and qualitatively judging the development toxicity of the tested substance by directly observing the existence and quantity of the food side nematodes withnaked eyes. The time from exposure to index detection is about 24 hours, the index detection time is shortened, naked eye observation and microscopic observation are integrated, and the detection result has high accuracy and reliability.
Owner:TONGJI UNIV +1
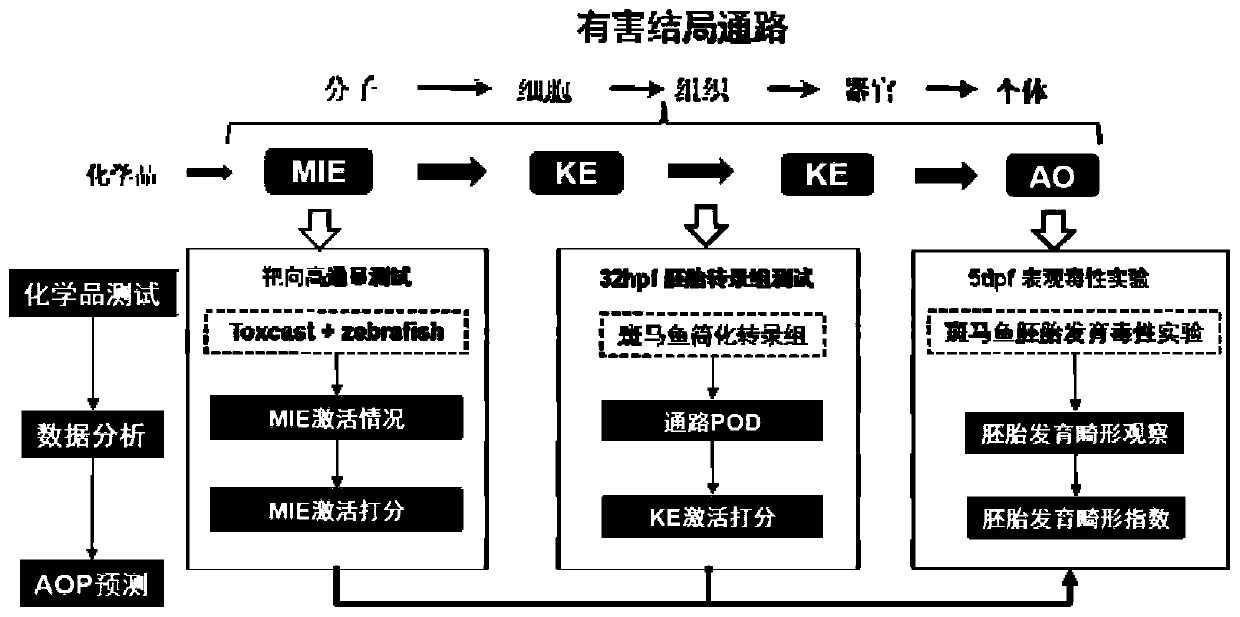
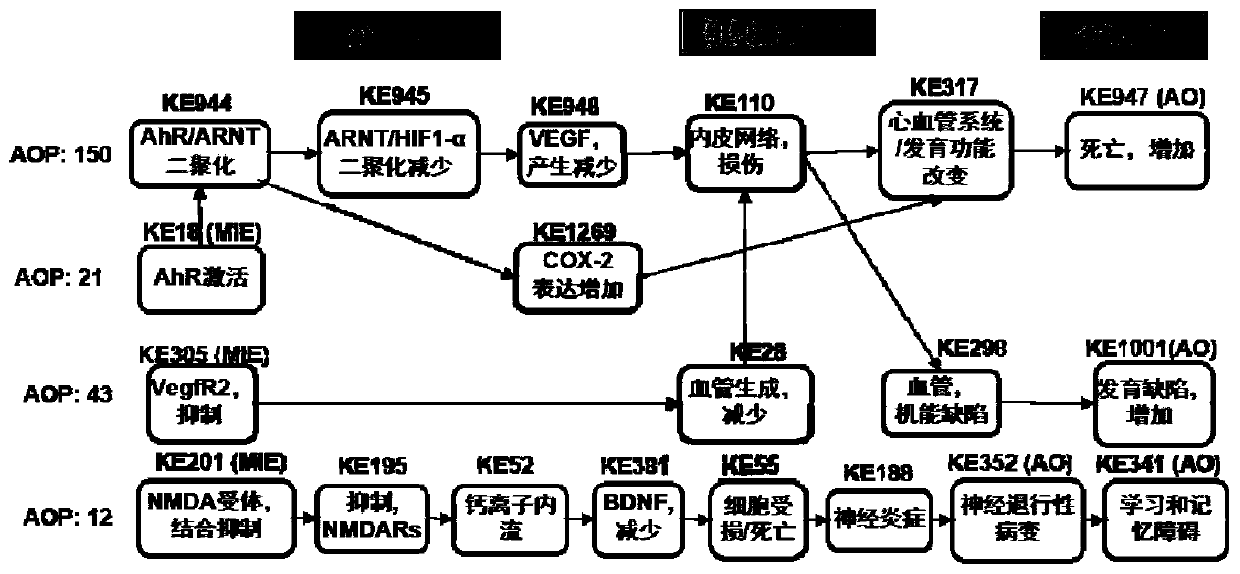
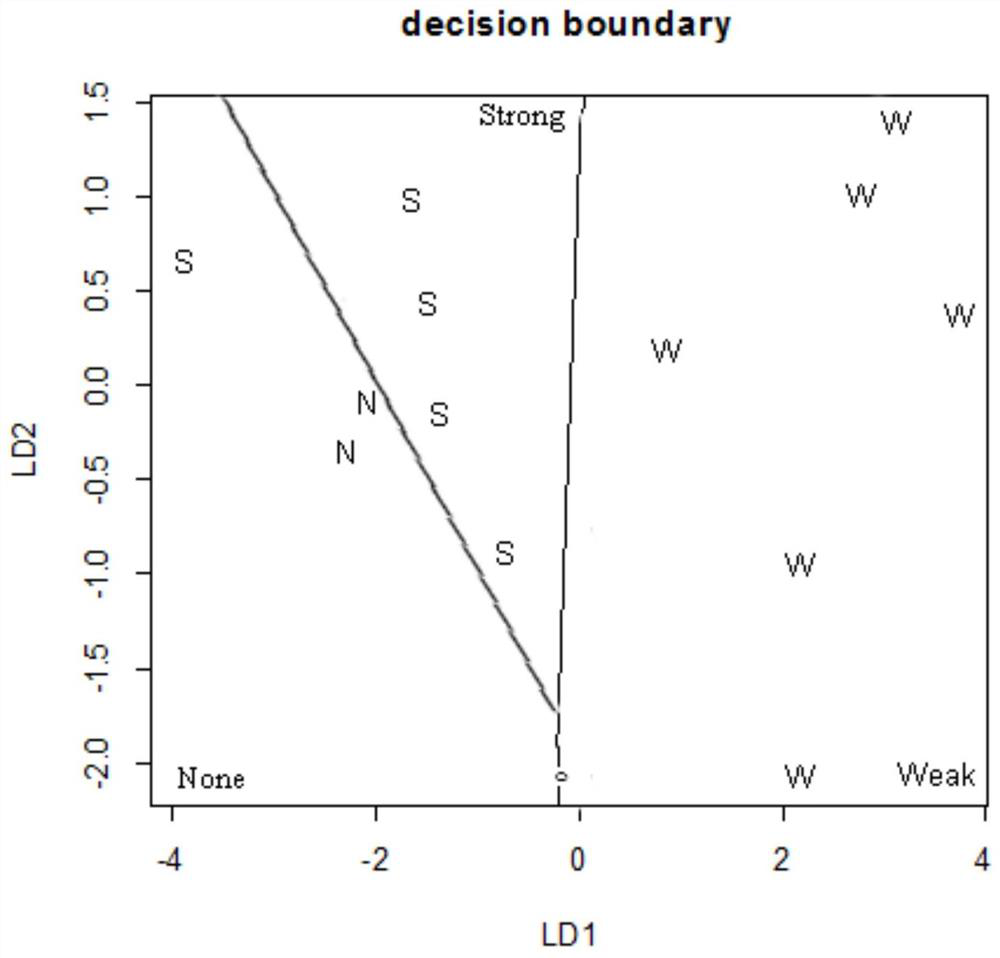
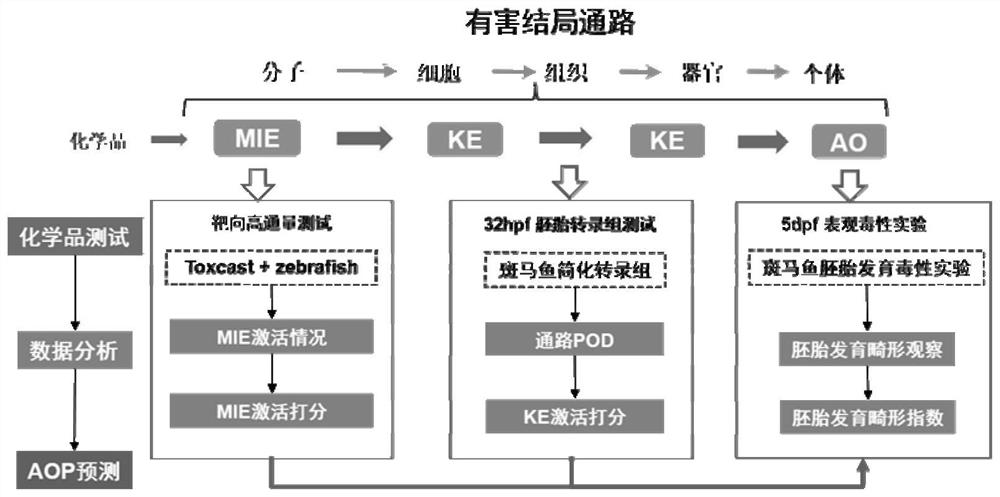
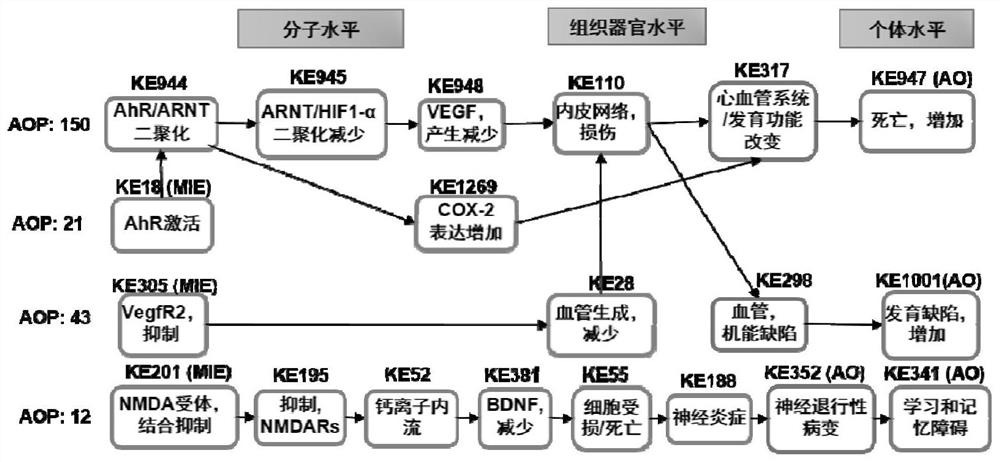
Method for predicting embryonic development toxicity of chemicals based on dose-effect simplified transcriptome
ActiveCN110415770AImprove discriminationGood forecastChemical property predictionCheminformatics data warehousingLife stageFish development
The invention discloses a calculation method for predicting the embryonic development toxicity of chemicals based on dose-effect simplified transcriptome, and belongs to the field of predictive toxicology. According to the calculation method, high-throughput testing data is systematically integrated for grading calculation to predict the embryonic toxicity of the chemicals and identify the key toxicity mechanism of the chemicals. In the calculation method, relevant evidence between key events (KE) is taken as the basis, and the high-throughput molecular testing data like the dose-effect simplified transcriptome is utilized to conduct activation grading calculation on an adverse outcome pathway (AOP) related to zebra fish development. The calculation method is more efficient and sensitive than a traditional embryonic development toxicity test and realizes efficient toxicity prediction based on the chemical toxication mechanism; by determining the influence of the low-dose chemicals on the early embryo transcriptional level, the developmental toxicity effect in a longer life stage can be predicted, the toxication mechanism of the chemicals can be identified, and the chemicals with different toxication modes can be distinguished.
Owner:NANJING UNIV
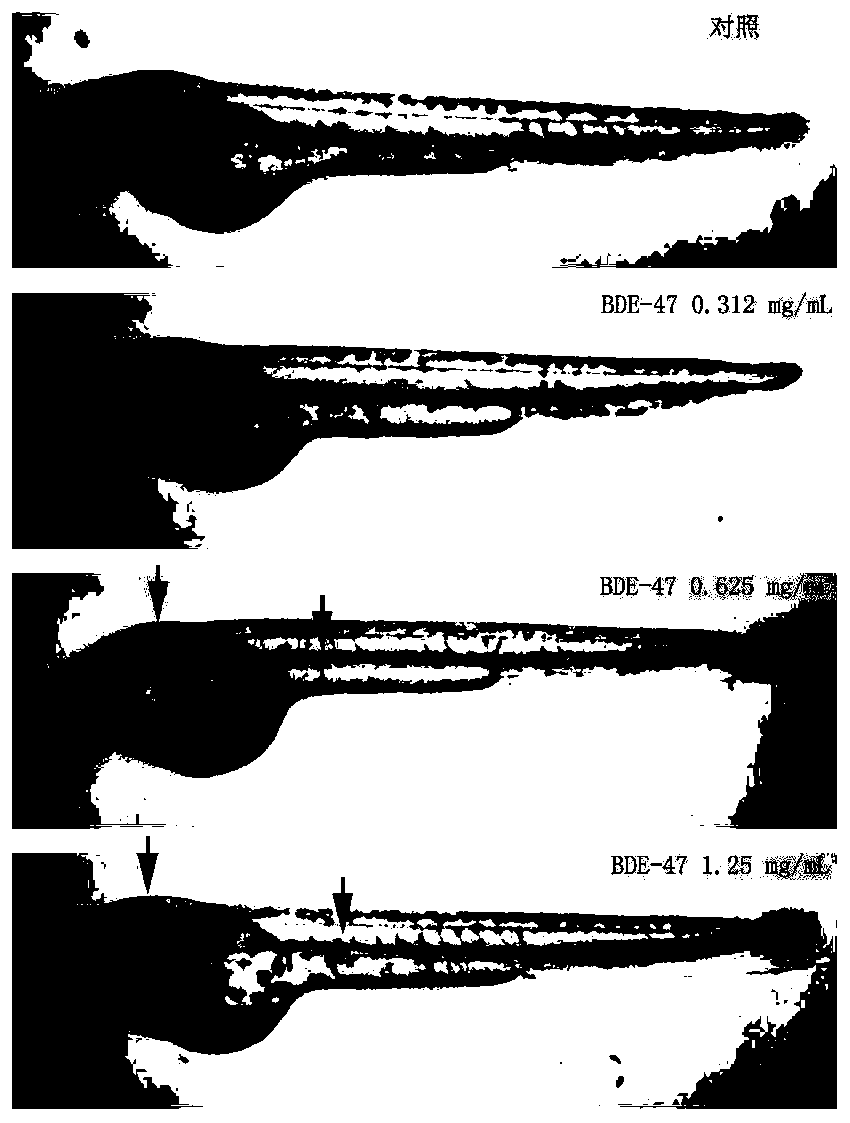
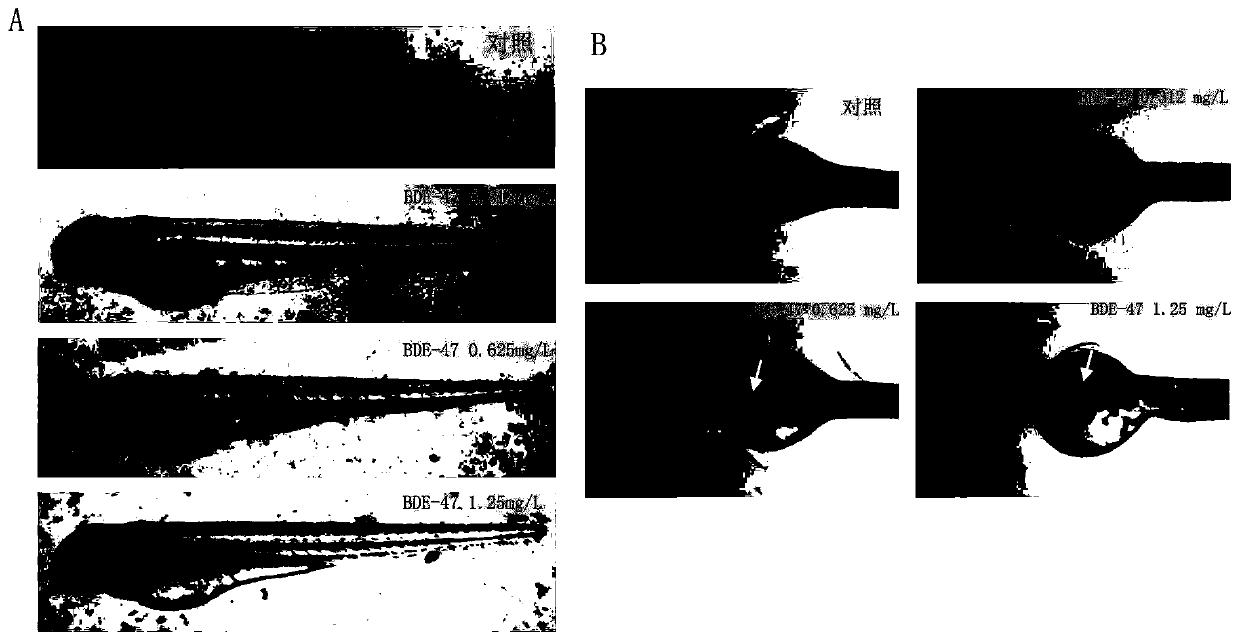
Method of evaluating developmental toxicity of polybrominated diphenyl ethers
InactiveCN110470657AImprove detection efficiencyImprove intuitivenessAnalysis by subjecting material to chemical reactionDiphenyl etherBrominated Diphenyl Ethers
The invention provides a method of evaluating the developmental toxicity of polybrominated diphenyl ethers, which relates to the technical field of toxicology. The method includes the following steps:normal developing zebrafish embryos are placed in polybrominated diphenyl ether solutions of different concentrations for cultivation for 44 to 68 h as an experiment group, a blank control group is set at the same time, and the zebrafish embryo pigment change is compared; and the embryo pigment concentration in the experiment group is significantly reduced in comparison with the blank control group, suffering from the developmental toxicity of polybrominated diphenyl ethers is proved. The method provided in the invention uses the zebrafish as a test object to detect the polybrominated diphenyl ethers, a large number of test animals with good homogeneity can be acquired quickly, the test cost is reduced, the symptoms of poisoning can be observed easily, the fish pigment change can be observed intuitively in real time, and the efficiency, the intuitiveness and the accuracy of polybrominated diphenyl ether detection are improved.
Owner:HUAIYIN TEACHERS COLLEGE
Method for rapidly evaluating potential teratogenicity of Chinese patent medicines by using embryo of zebrafish
The invention relates to a method for rapid evaluation and testing of the potential teratogenicity of Chinese patent medicines. The method is characterized in that the embryo of zebrafish is taken as a toxicity model and exposed to an aqueous solution of a Chinese patent medicine; whether the embryo of zebrafish has developmental toxicity during development under the stimulation of the Chinese patent medicine is observed; and whether the Chinese patent medicine has potential teratogenicity is determined according to the indexes of toxicity of the Chinese patent medicine to the embryo of zebrafish. With the method, the toxicity of a plurality of frequently used gynecological Chinese patent medicines are successfully evaluated and the potential teratogenicity of the gynecological Chinese patent medicines are effectively tested, so significant guidance is provided for safe clinical usage of the Chinese patent medicines.
Owner:SUN YAT SEN UNIV
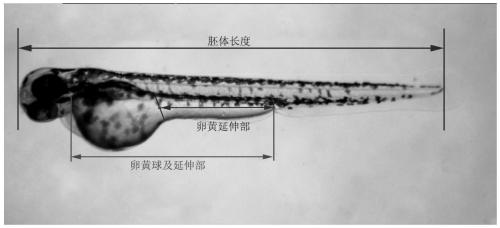
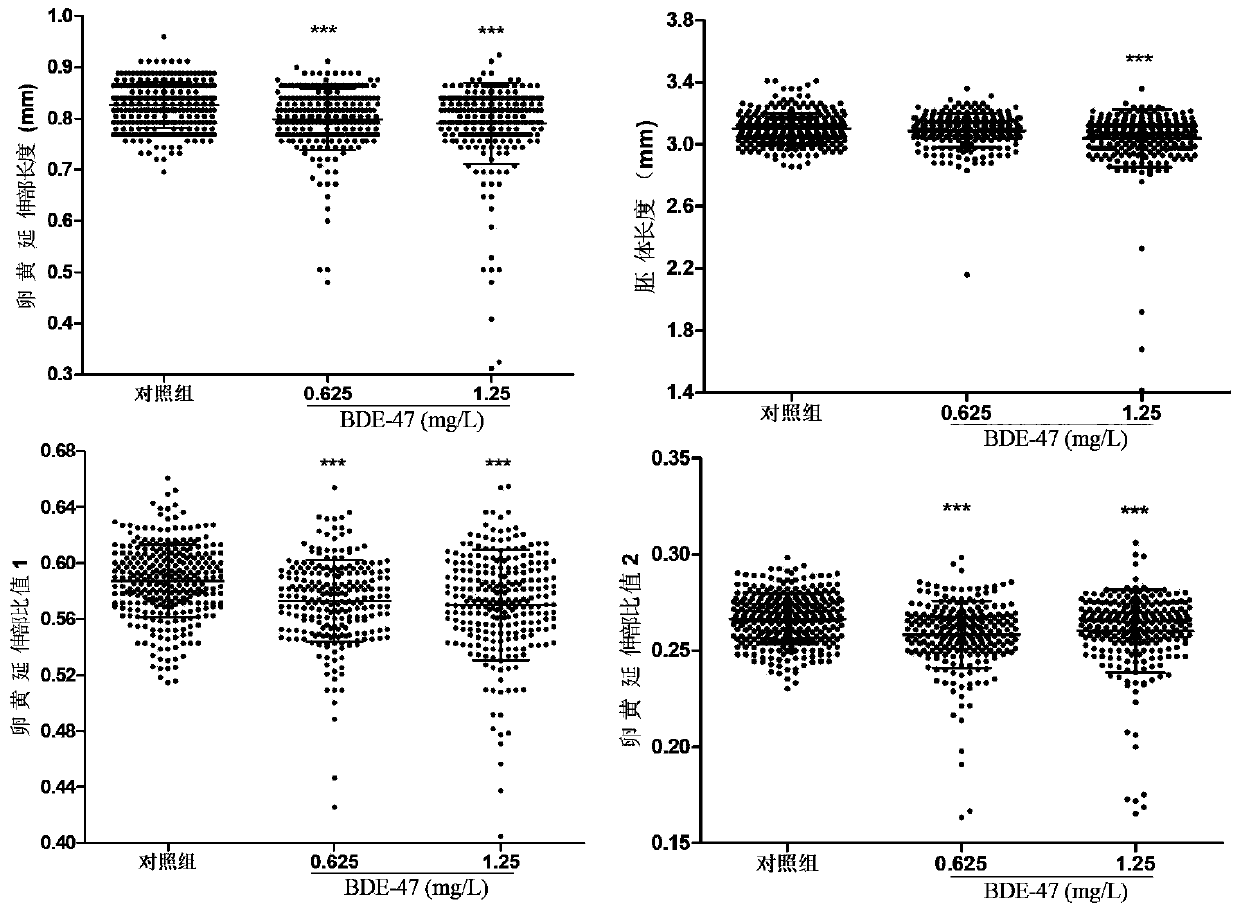
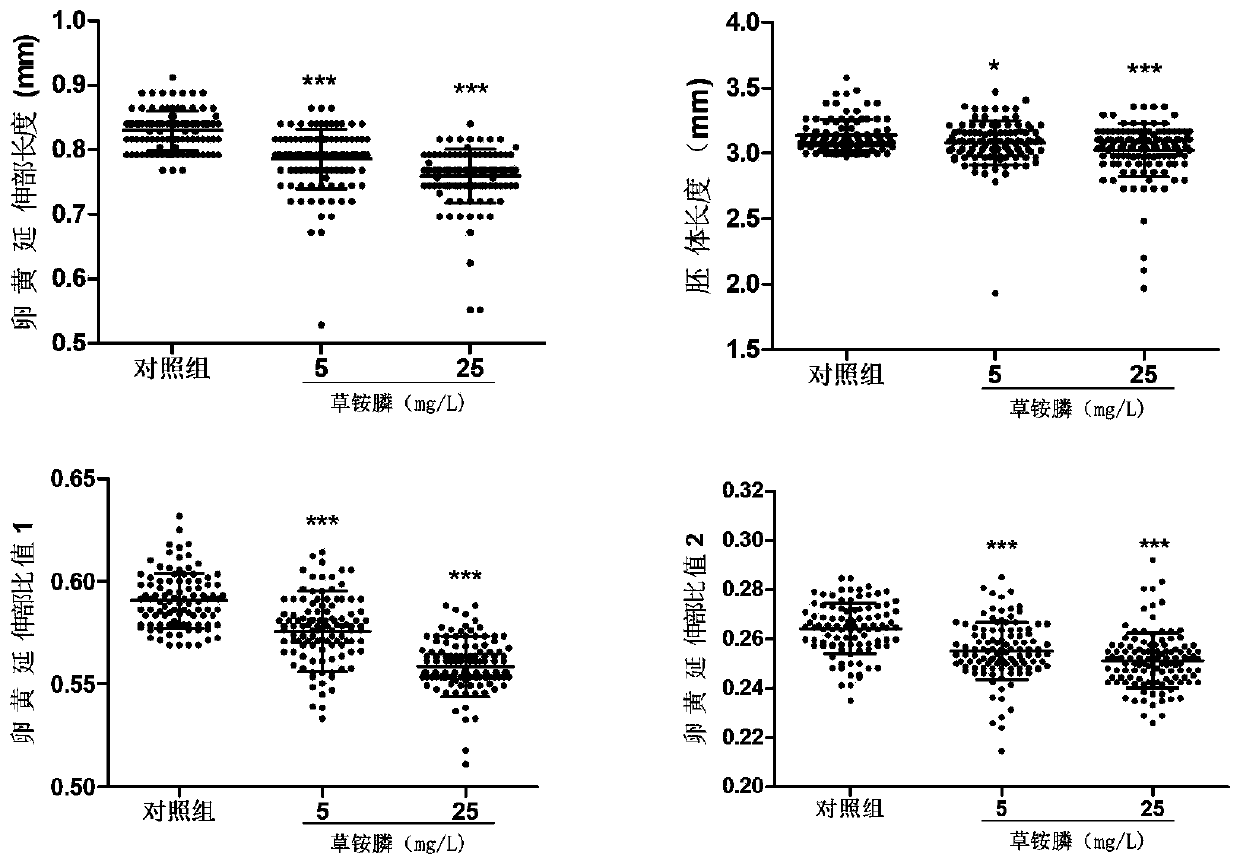
Method for evaluating developmental toxicity of environmental pollutants by utilizing lengths of egg yolk extension parts of Danio rerio
PendingCN111471739ADelayed developmental featuresHigh degree of developmental inhibitionMicrobiological testing/measurementMaterial analysisYolkEmbryo
The invention provides a method for evaluating the developmental toxicity of environmental pollutants by utilizing the lengths of egg yolk extension parts of Danio rerio, and belongs to the technicalfield of environmental detection technology and toxicity research. The method comprises the steps of exposing Danio rerio embryos developed to 4-5 hpf to the environmental pollutants or a control culture solution, and counting the lengths of the egg yolk extension parts of the embryos, the ratio of the lengths of the egg yolk extension parts to the total length of egg yolk spheres and the extension parts or the ratio of the lengths of the egg yolk extension parts to the lengths of the embryos when the Danio rerio embryos are developed to 60-84 hpf; and when any one of the lengths of the yolk extension parts, the ratio of the lengths of the yolk extension parts to the total length of the yolk spheres and the extension parts, and the ratio of the lengths of the yolk extension parts to the lengths of the embryos of an environmental pollutant group is obviously different from that of a control group, indicating that the environmental pollutants have developmental toxicity. Compared with egg yolk sphere development and embryo development, the development of the yolk extension parts is more sensitive to the toxicity of the environmental pollutants, the developmental retardation toxicityeffect of lower-dose environmental pollutants can be effectively evaluated, and meanwhile, the method is simple, convenient and rapid to operate and high in detection sensitivity.
Owner:HUAIYIN TEACHERS COLLEGE
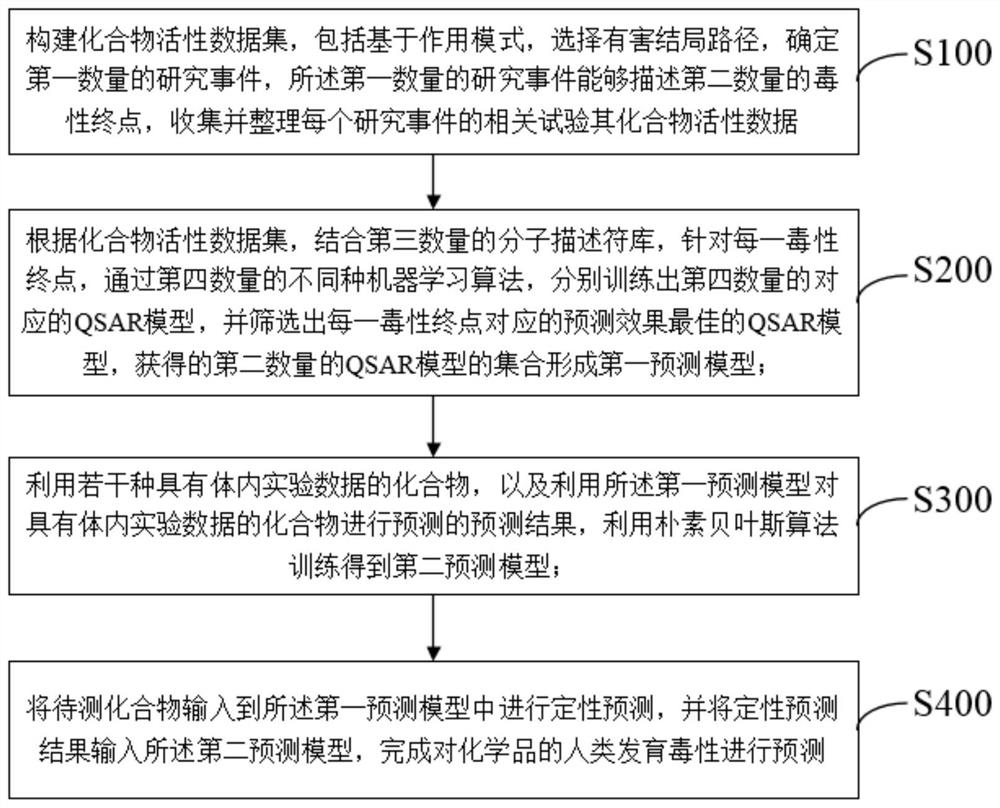
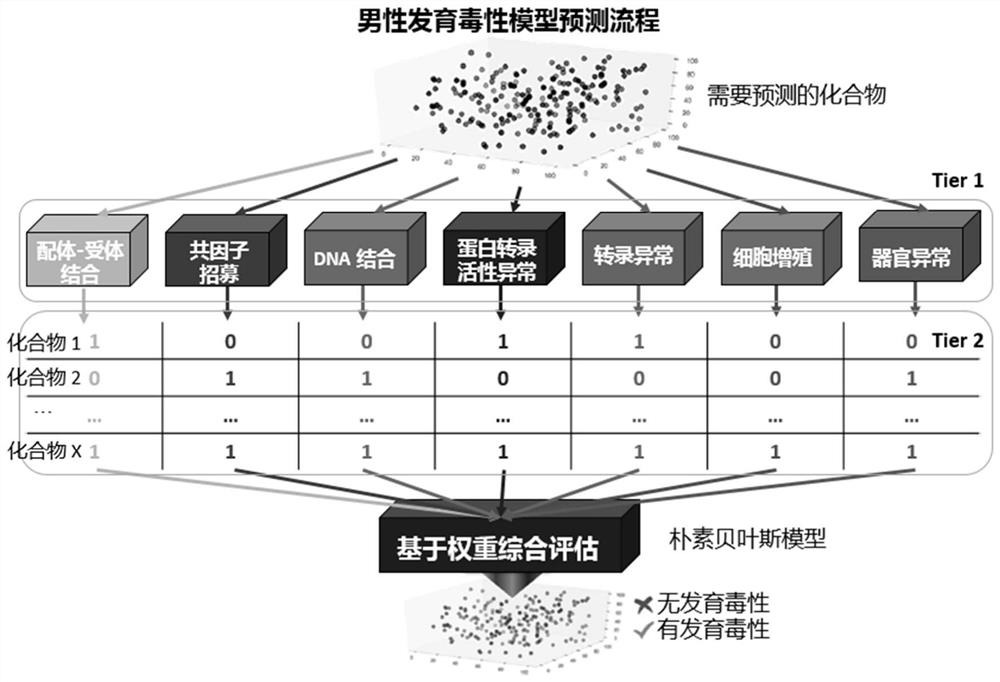
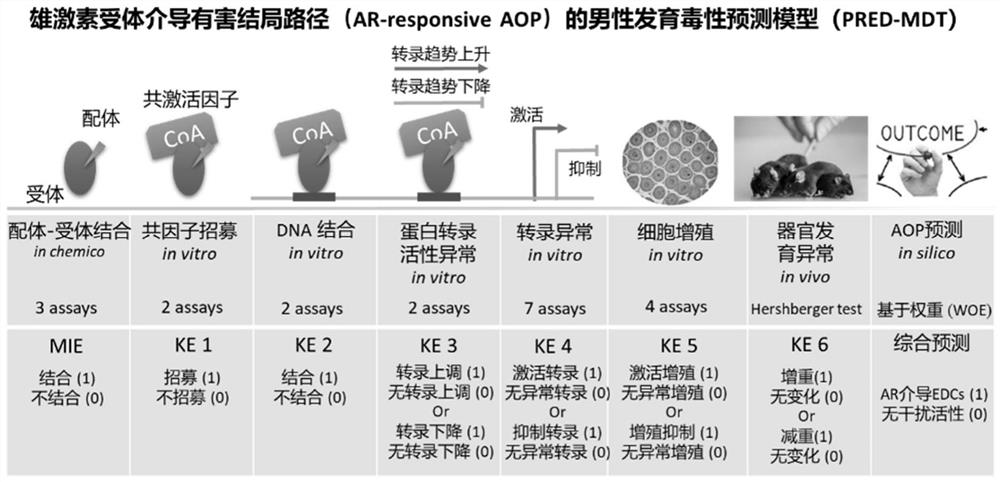
Human developmental toxicity prediction method based on action mode
PendingCN113409899AJudgment of developmental toxicityChemical machine learningMedical referencesData setChemical compound
The invention discloses a human developmental toxicity prediction method based on an action mode, and belongs to the field of human developmental toxicity virtual screening and activity prediction of chemicals. The method comprises the following steps: constructing a compound activity data set, selecting a harmful outcome path based on a human developmental toxicity action mode, and collecting research events and activity data thereof on a signal path of the harmful outcome path; constructing a first prediction model based on the data set; training by using a naive Bayesian algorithm to obtain a second prediction model by using a plurality of compounds with in-vivo experimental data and a prediction result obtained by predicting the compounds with the in-vivo experimental data by using the first prediction model; and inputting a to-be-detected compound into the first prediction model for qualitative prediction, and inputting a qualitative prediction result into the second prediction model to complete the prediction of the human developmental toxicity of the chemical. The method provided by the invention can be used for high-throughput screening of potential action mode-based human developmental toxic chemicals.
Owner:NANJING UNIV
Test method of developmental toxicity of silkworms by environmental estrogen
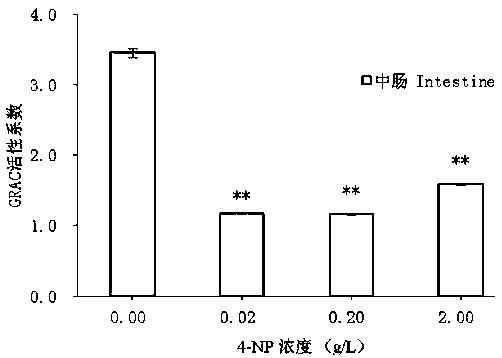
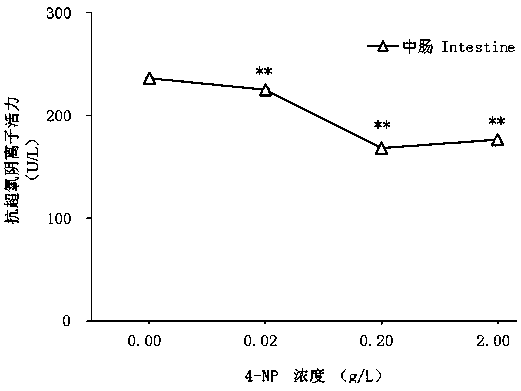
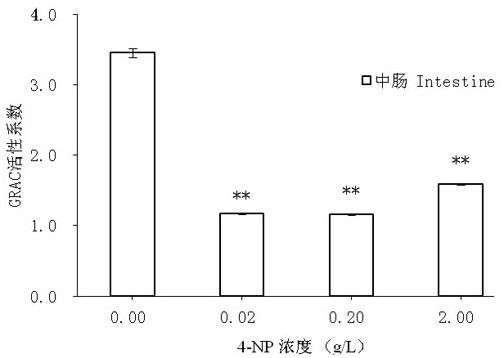
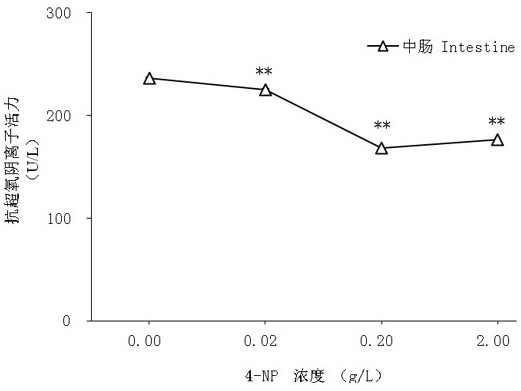
ActiveCN109557319AFast detection methodThe detection method is simpleBiological testingSuperoxideGlutathione reductase activity
The invention discloses a test method of developmental toxicity of silkworms by environmental estrogen. The method includes following steps: firstly, injecting a test sample containing a series of concentrations to 5-age silkworm larvae through 2-hour starvation treatment by employing oral gavage; after 20 minutes, performing anatomy on the silkworms through gavage, and taking tissues and organs;determining the developmental toxicity of the silkworms by the test sample through comparison of toxic endpoints of an infected group and a control group, wherein the toxic endpoints comprise a glutathione reductase activity coefficient and an anti-superoxide anion free radical activity of midguts of the silkworms; and researching the influence of the environmental estrogen 4-nonylphenol on the development of the midguts of the silkworms in a larva stage to determine a dose-effect relation between the environmental estrogen and the developmental toxicity of the silkworms in the larva stage. Byemploying the technical scheme, multiple biological effect endpoints can be generated for the environmental estrogen nonylphenol, potential risks of the silkworms exposed in an estrogen environmentalconcentration level are evaluated, and the method is a relatively fast, simple and sensitive detection method.
Owner:SUZHOU UNIV OF SCI & TECH
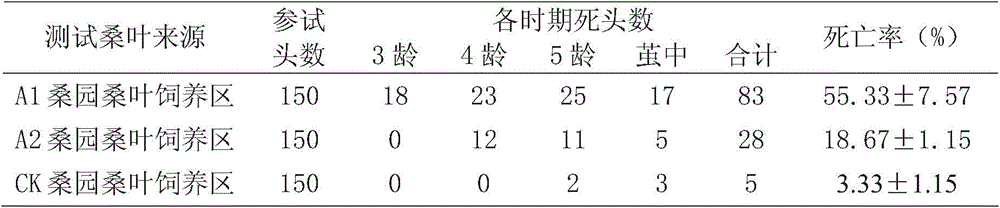
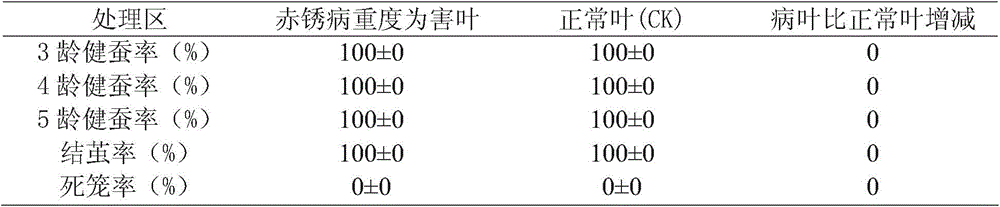
Method for detecting toxicity and safety of contaminated mulberry leaves
Owner:广西壮族自治区蚕业技术推广总站
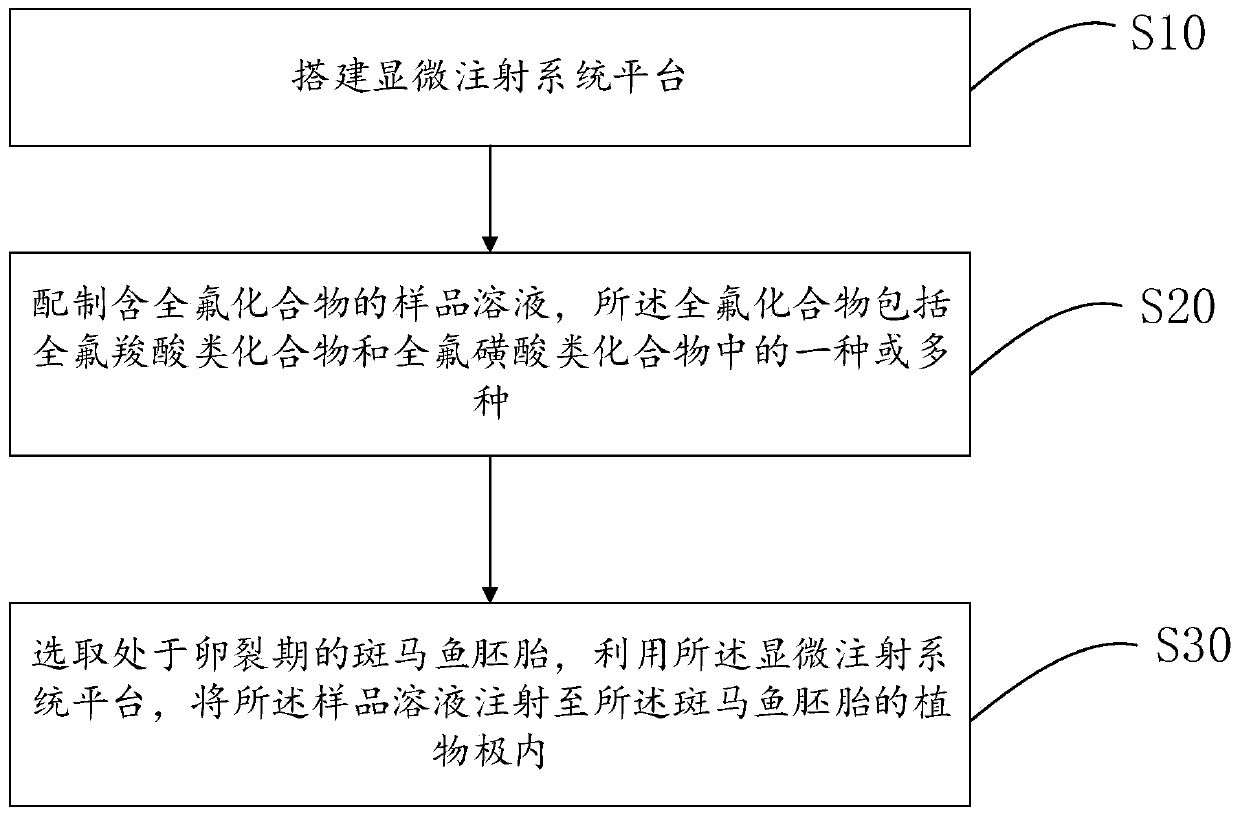
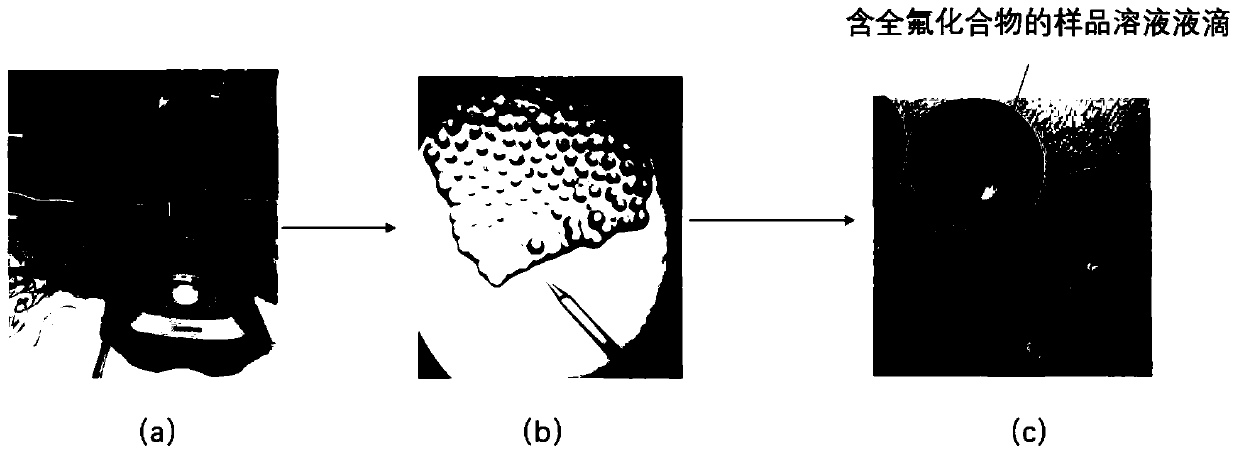
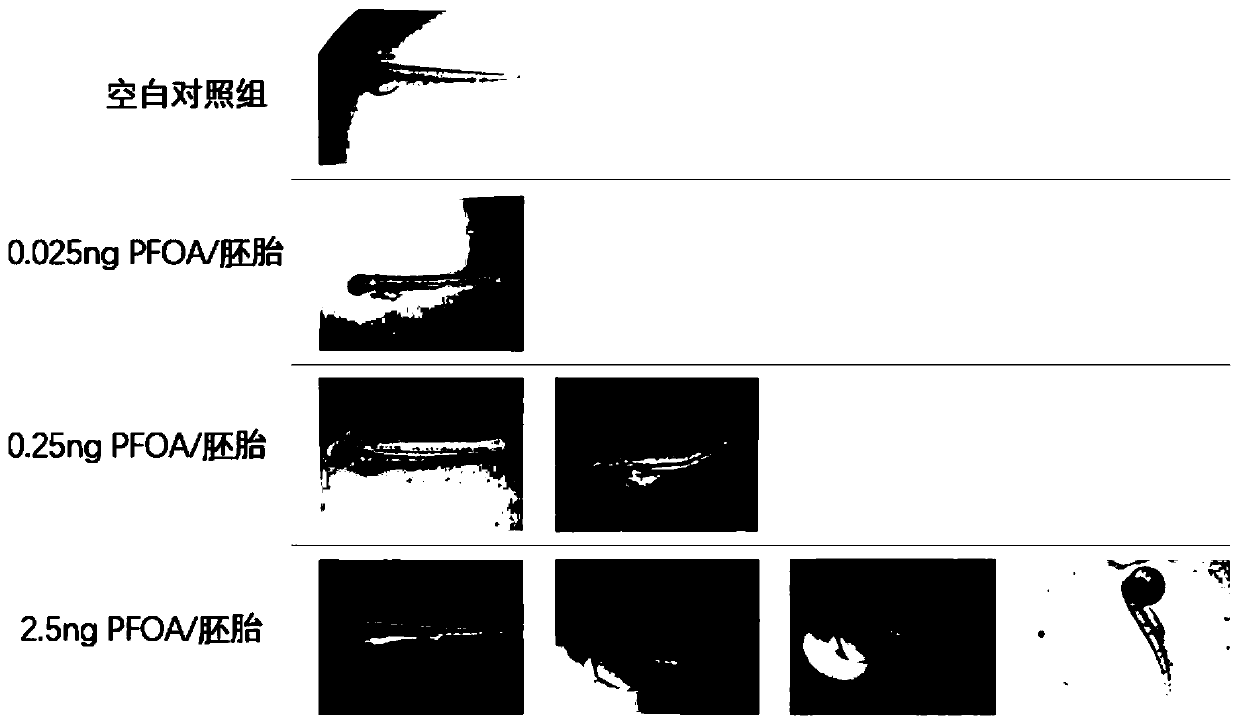
Construction method and application of zebra fish embryo perfluorinated compound internal exposure model
InactiveCN111040990AImprove accuracyEfficient acquisitionMicrobiological testing/measurementCell culture active agentsBiotechnologyFish embryo
Owner:SHENZHEN INST OF ADVANCED TECH
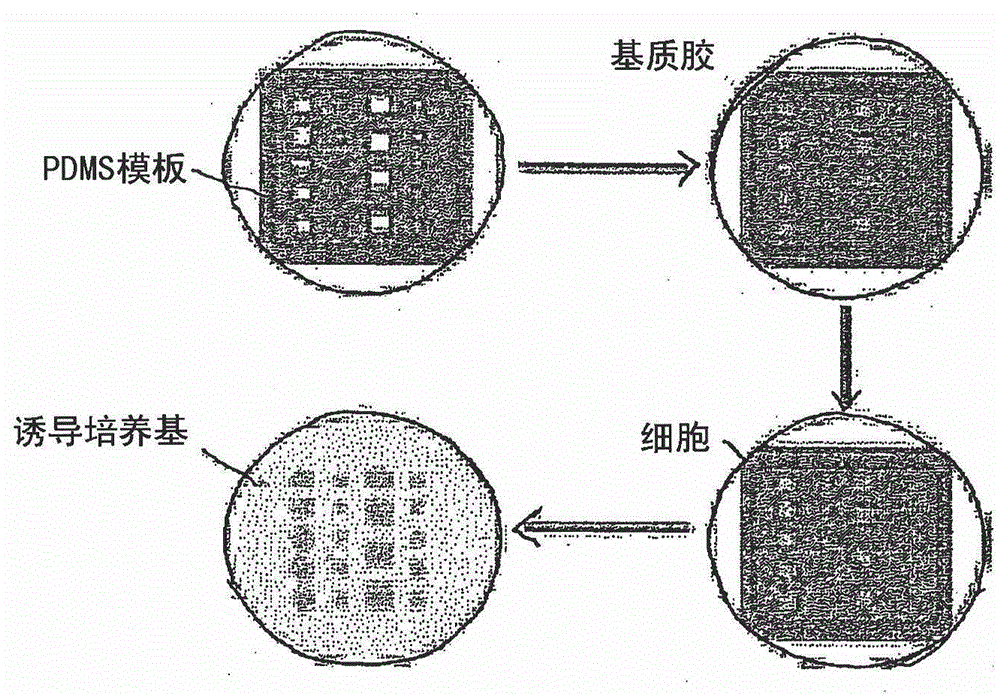
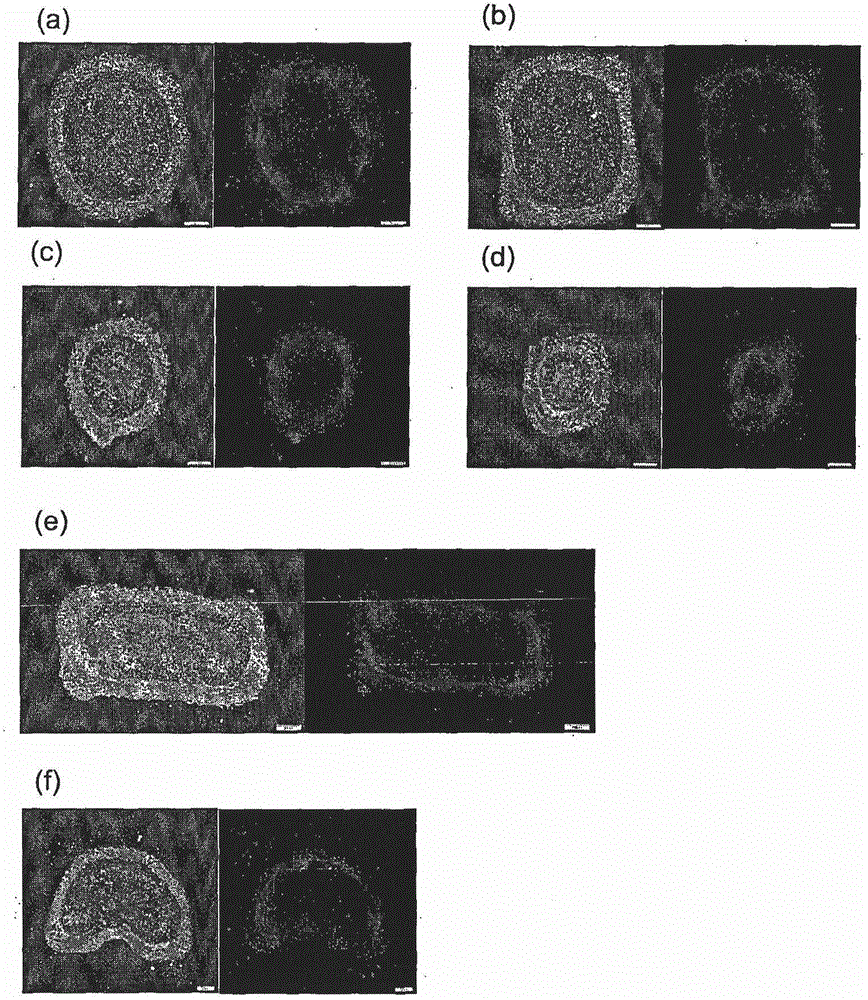
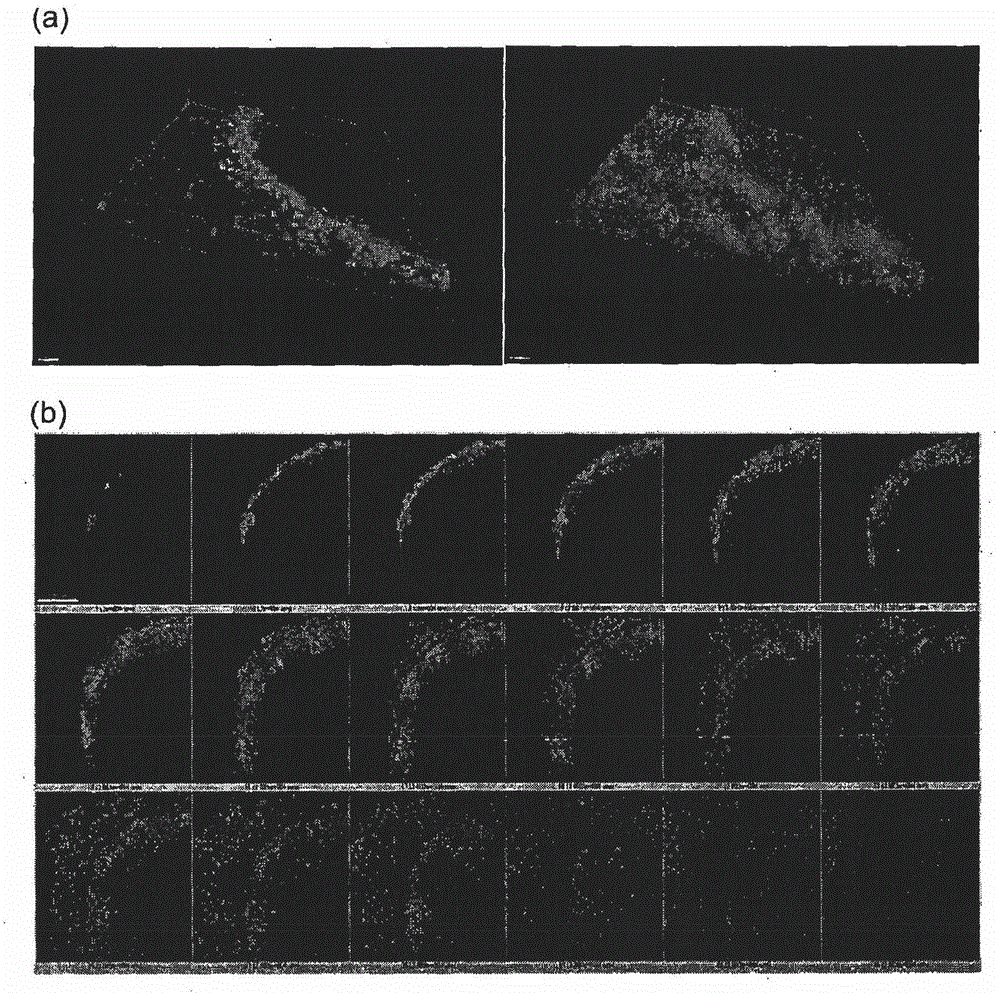
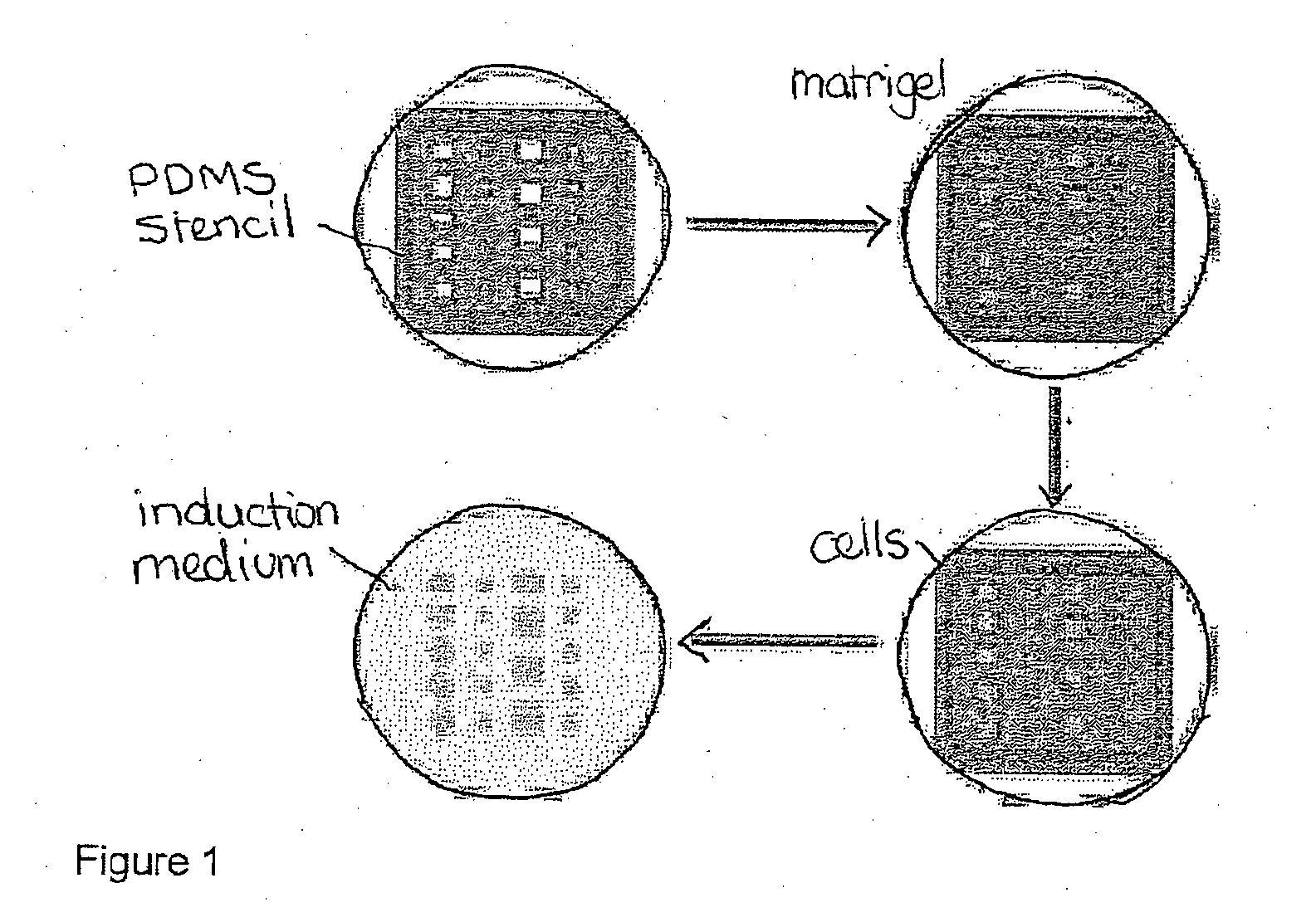
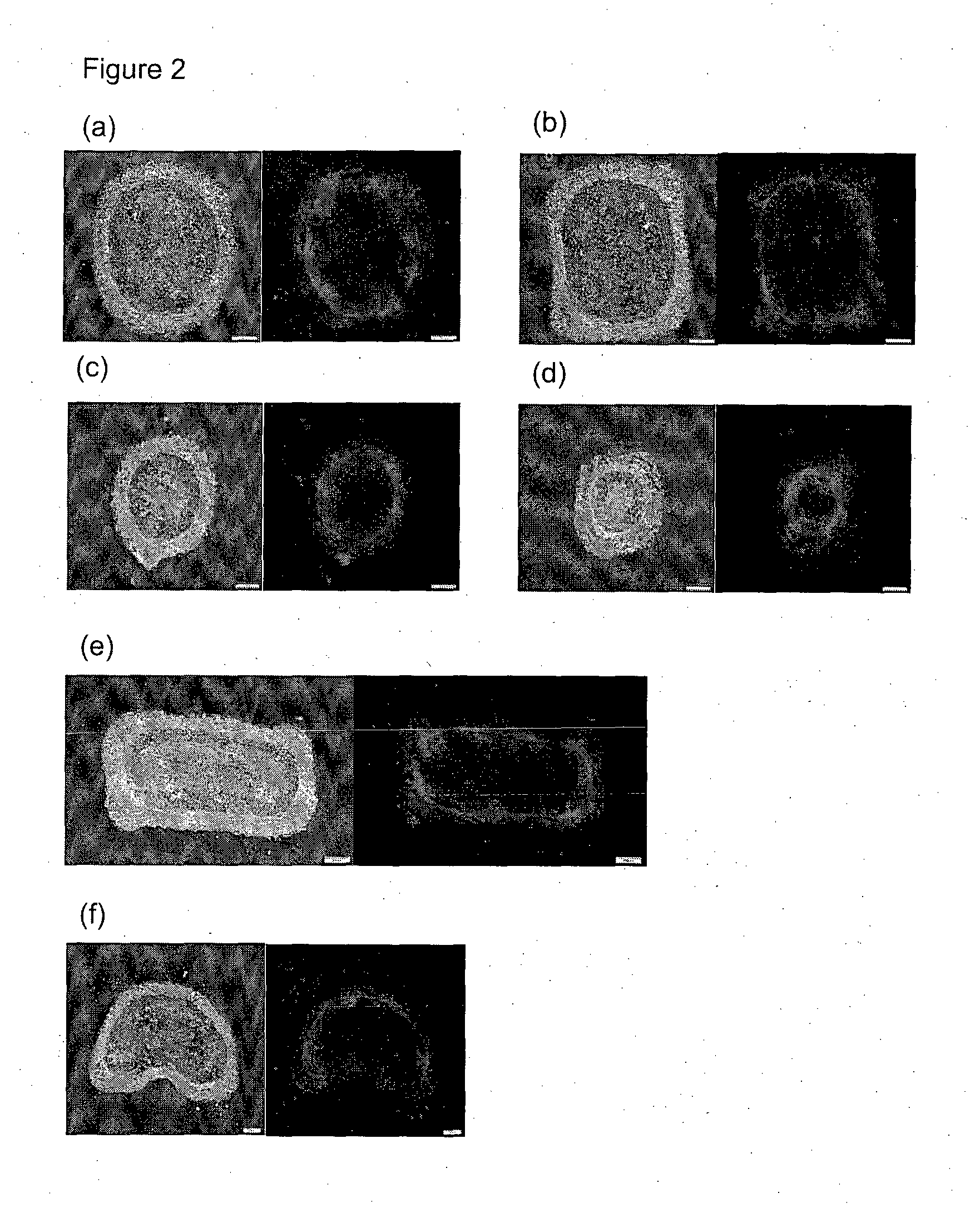
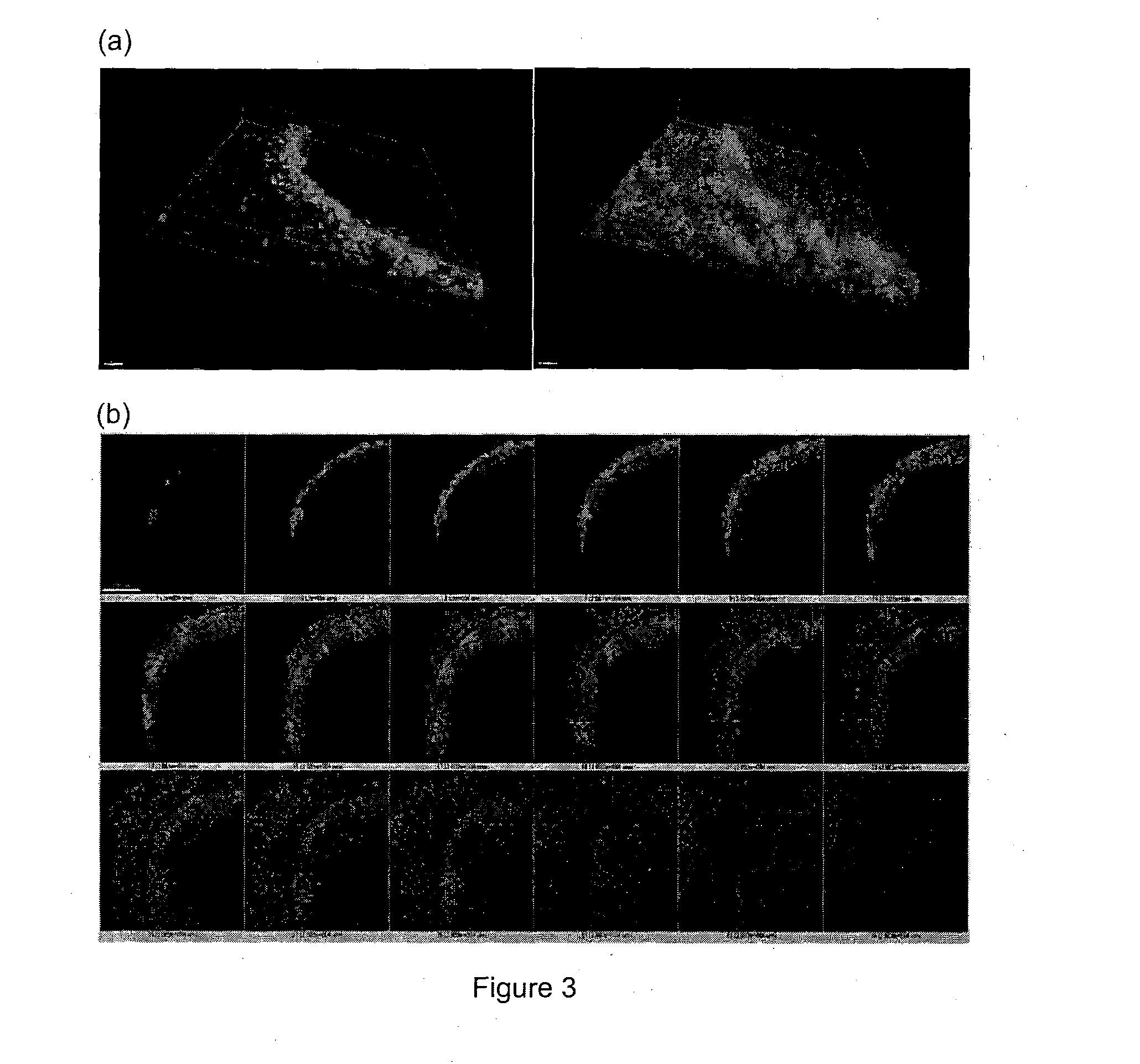
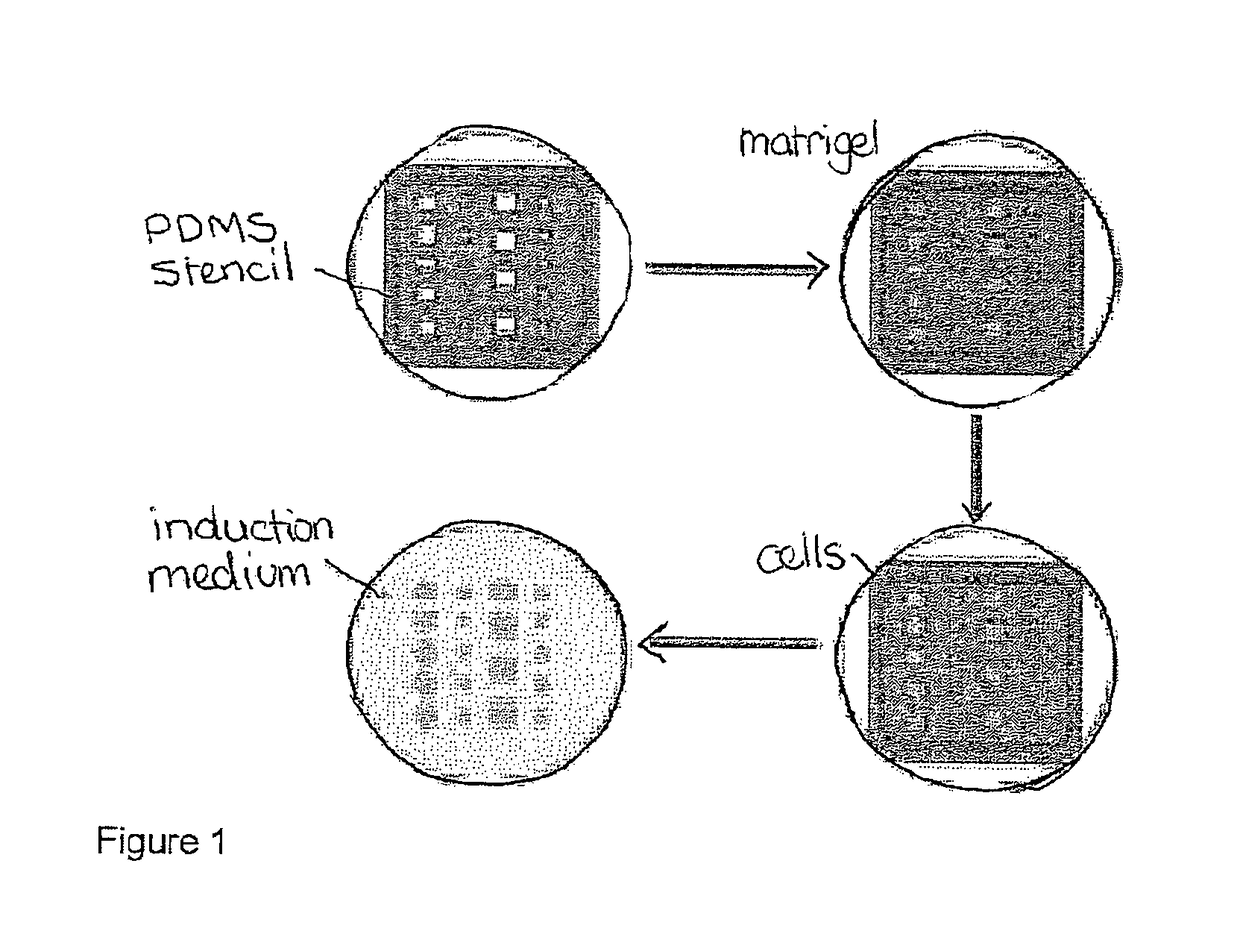
Method and system for in vitro developmental toxicity testing
A method and system of in vitro developmental toxicity testing comprising the steps of micropatterning an extracellular matrix; growing embryonic stem cells on the micropatterned extracellular matrix in the presence of mesoendermal induction and testing for change of the geometrical mesoendoderm structure in the presence or absence of a test compound wherein (1) a decrease in mesoendodermal differentiation and / or (2) a change in morphology of the geometrical mesoendoderm structure in the presence of the test compound compared to cells in the absence of the test compound indicates that the test compound is a developmental toxic agent.
Owner:AGENCY FOR SCI TECH & RES
Predicting human developmental toxicity of pharmaceuticals using human stem-like cells and metabolomic ratios
ActiveUS20180128814A1Material analysis using wave/particle radiationSkeletal/connective tissue cellsMetaboliteFold change
Owner:STEMINA BIOMARKER DISCOVERY
Method for evaluating toxicity of cosmetic basic raw materials to infant development by using zebra fish
PendingCN114859029AConvenient detection workEasy to filter jobsClimate change adaptationBiological testingBiotechnologyAnimal science
The invention provides a method for evaluating the toxicity of cosmetic base materials to infant development by using zebrafish, which comprises the following steps: (1) equally selecting zebrafish embryos which develop for 24-72 hours from fertilization, exposing the zebrafish embryos in a plurality of groups of embryo culture solutions containing base materials with different gradient concentrations, and culturing for 72 hours, selecting a basic raw material concentration which has a hatching rate of more than 95% and does not cause zebrafish body organ injury and development malformation as a safe concentration; (2) averagely dividing zebrafish embryos developed for 24-72 hours from fertilization into a control group and a plurality of treatment groups, exposing the zebrafish in the treatment groups in an embryo culture solution with a safe-concentration basic raw material for culturing, and after culturing for 72 hours, measuring and evaluating heart rate, development malformation degree, ROS oxidative stress and growth and development related gene expression conditions, so as to obtain the zebrafish embryos. And evaluating the developmental toxicity of the to-be-detected basic raw material. The method disclosed by the invention is simple and convenient to operate, beneficial to large-batch raw material detection and screening work, and relatively high in accuracy.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Method for evaluating developmental toxicity of environmental pollutant H2S by using zebra fish
PendingCN113702376AAccurate evaluationShort cycleMaterial analysis by optical meansJuvenile fishFishery
The invention discloses a method for evaluating developmental toxicity of an environmental pollutant H2S by using zebrafish, which comprises the following steps of: culturing zebrafish fertilized eggs in H2S environments with different concentrations, performing statistics on hatching of the zebrafish fertilized eggs by H2S with different concentrations and survival rate of juvenile fishes of the zebrafish fertilized eggs, and observing forms such as spines, postures and swimming bladders of the juvenile fishes, so as to evaluate the developmental toxicity of the environmental pollutant H2S. The method for evaluating the developmental toxicity of the environmental pollutant H2S by using the zebrafish is simple and convenient to operate, short in evaluation period, efficient and intuitive. The evaluation period can be completed within 10 days, the operation is simple, convenient, rapid and efficient, and the cost can be greatly reduced; the evaluation result of the evaluation method provided by the invention not only can provide a certain reference for the developmental toxicity of the environmental pollutant H2S in the water body to other water body organisms, but also can provide a certain data support and reference for the control of H2S in the water body.
Owner:WENZHOU UNIVERSITY
A method for evaluating the growth and development toxicity of triazole pesticides using Drosophila melanogaster
ActiveCN110150236BGrowth and development toxicity hasAccurate evaluationAnimal feeding stuffAccessory food factorsDrosophila ornatifronsAnimal science
Owner:BEIJING FORESTRY UNIVERSITY
A method for detecting developmental toxicity of chemicals
The invention provides a method for detecting the developmental toxicity of chemicals, which at least includes the following steps: respectively inducing undifferentiated human embryonic stem cells to differentiate into cardiomyocytes under the culture conditions of the chemical to be tested and without the chemical to be tested; There is the expression level of MYL4 in the cardiomyocytes under the culture conditions of the chemicals to be tested and without the chemicals to be tested; The change in the expression level of MYL4 in is ID; according to the value of ID, combined with the Fisher discriminant function equation, the developmental toxicity of the chemical to be tested can be judged. The invention uses human embryonic stem cells to establish a developmental toxicity test model for the first time, and the developmental toxicity information of the compound can be obtained through in vitro tests in a relatively short period of time; the invention can quickly detect and prompt whether a certain chemical has developmental toxicity information for humans.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
A method for predicting embryonic developmental toxicity of chemicals based on dose-response simplified transcriptome
ActiveCN110415770BImprove discriminationGood forecastChemical property predictionCheminformatics data warehousingBiotechnologyEmbryo
The invention discloses a calculation method for predicting the embryonic developmental toxicity of chemicals based on dose-effect simplified transcriptome, and belongs to the field of predictive toxicology. The invention predicts the embryotoxicity of chemicals and identifies the key toxicity mechanism of chemicals by systematically integrating high-throughput test data for scoring calculation. The calculation method of the present invention is based on the correlation evidence between key events (KE), and utilizes dose-effect simplified transcriptome and other high-throughput molecular test data to perform activation scoring calculation on zebrafish development-related harmful outcome pathway (AOP). The invention is more efficient and sensitive than the traditional embryonic developmental toxicity test, realizes efficient toxicity prediction based on the mechanism of chemical toxicity, and can predict the development of longer life stages through the influence of low-dose chemicals on the transcription level of early embryos Toxic effect, identify the toxic mechanism of chemicals, and distinguish chemicals with different toxic modes.
Owner:NANJING UNIV
Method and system for in vitro developmental toxicity testing
A method and system of in vitro developmental toxicity testing comprising the steps of micropatterning an extracellular matrix; growing embryonic stem cells on the micropatterned extracellular matrix in the presence of mesoendermal induction and testing for change of the geometrical mesoendoderm structure in the presence or absence of a test compound wherein (1) a decrease in mesoendodermal differentiation and / or (2) a change in morphology of the geometrical mesoendoderm structure in the presence of the test compound compared to cells in the absence of the test compound indicates that the test compound is a developmental toxic agent.
Owner:AGENCY FOR SCI TECH & RES
Method and system for in vitro developmental toxicity testing
Owner:AGENCY FOR SCI TECH & RES
A kit and application for screening drug developmental toxicity based on c/ebpα and igf1r genes
ActiveCN107841556BReliable and reliableAdaptableMicrobiological testing/measurementCell lysatesPromoter
The invention relates to a kit for screening drug developmental toxicity based on a C / EBP[alpha]-IGF1R gene and an application thereof. The kit contains: primers for detecting expression of C / EBP[alpha] and IGF1R gene mRNA; drug acting object mammalian cell lines or differentiated cells, and cell lines or differentiated cells having a plasmid containing an IGF1R promoter and a luciferase reportergene transferred into cells; a cell lysate; and luciferase. The determination standard of the kit comprises improvement of intracellular C / EBP[alpha] gene expression of a to-be-screened compound, reduction of IGF1R gene expression, and reduction of the activity of the luciferase reporter gene in the cells containing the IGF1R promoter-reporter gene, and the to-be-screened compound is suggested tohave developmental toxicity. The kit is novel, efficient and reliable, and can be used for high-throughput drug screening, and is of great significance for rapid detection of drugs and other compoundswith developmental toxicity.
Owner:WUHAN UNIV
A method for testing the developmental toxicity of environmental estrogen to silkworm
ActiveCN109557319BFast detection methodThe detection method is simpleBiological testingAnimal scienceGlutathione reductase activity
The invention discloses a method for testing the developmental toxicity of environmental estrogens to silkworms, which comprises the following steps: firstly, the 5th instar silkworm larvae containing a series of concentrations of test substances are starved for 2 hours by oral gavage injection; Stomach of the silkworm was dissected to obtain tissues and organs. Toxicity endpoints include glutathione reductase activity coefficient and anti-superoxide anion free radical activity in silkworm midgut. The toxicity of the test substance to silkworm development was determined by comparing the toxicity endpoints of the exposure group and the control group. To study the effects of environmental estrogen 4‑nonylphenol on midgut development in silkworm larvae to determine its dose-effect relationship on developmental toxicity in silkworm larvae. By adopting the technical scheme of the invention, multiple biological effect endpoints can be produced on the environmental estrogen nonylphenol, and the potential risk of silkworm exposed to the estrogen environmental concentration level can be evaluated, which is a relatively fast, simple and sensitive detection method.
Owner:SUZHOU UNIV OF SCI & TECH
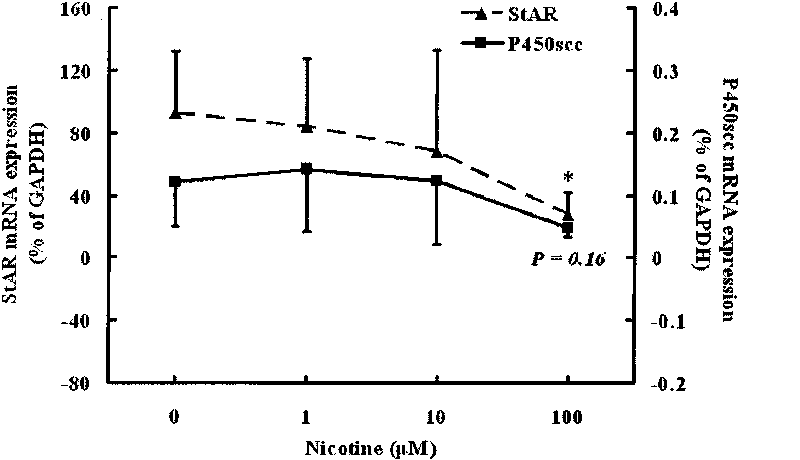
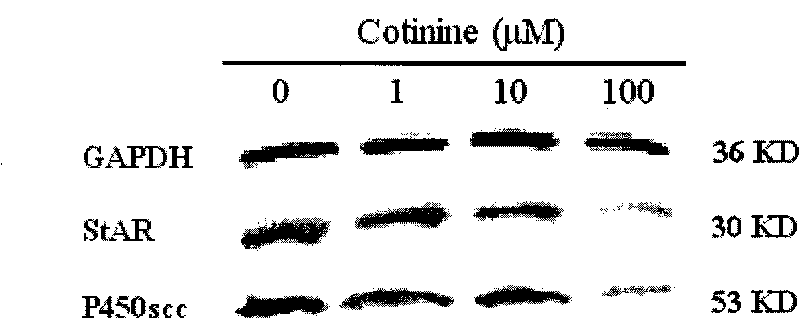
Application of embryo adrenal StAR genes in assessing developmental toxicity of chemicals
InactiveCN101712980ASimple methodFast wayMicrobiological testing/measurementBiological testingWHOLE ANIMALAdrenal cell
The invention discloses application of embryo adrenal StAR genes in assessing developmental toxicity of chemicals. An in vitro assessing system for the developmental toxicity of the chemicals is established, and comprises: 1) a human embryo adrenal cell in vitro culture method; 2) a mode of processing cells of chemical samples; and 3) a method for detecting StAR gene expression. The system is applied to verify that various allothigenes have the developmental toxicity so as to verify the embryo developmental toxicity of the positive chemicals which has existed in the whole animal level in the cell level. The in vitro assessing system can early and quickly assess the developmental toxicity of the chemicals so as to meet the multi-field requirements of research and development of new medicaments and assessment of environmental health.
Owner:WUHAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Kit for screening drug developmental toxicity based on C/EBP[alpha] and IGF1R gene and application thereof Kit for screening drug developmental toxicity based on C/EBP[alpha] and IGF1R gene and application thereof](https://images-eureka.patsnap.com/patent_img/1e089229-b0b1-4a44-b734-4bff2c4a823e/HDA0001515234750000011.png)
![Kit for screening drug developmental toxicity based on C/EBP[alpha] and IGF1R gene and application thereof Kit for screening drug developmental toxicity based on C/EBP[alpha] and IGF1R gene and application thereof](https://images-eureka.patsnap.com/patent_img/1e089229-b0b1-4a44-b734-4bff2c4a823e/HDA0001515234750000012.png)
![Kit for screening drug developmental toxicity based on C/EBP[alpha] and IGF1R gene and application thereof Kit for screening drug developmental toxicity based on C/EBP[alpha] and IGF1R gene and application thereof](https://images-eureka.patsnap.com/patent_img/1e089229-b0b1-4a44-b734-4bff2c4a823e/HDA0001515234750000013.png)