Methods to induce targeted protein degradation through bifunctional molecules
A bifunctional, determinant technology, applied or administered to animals or humans in the field of decoy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
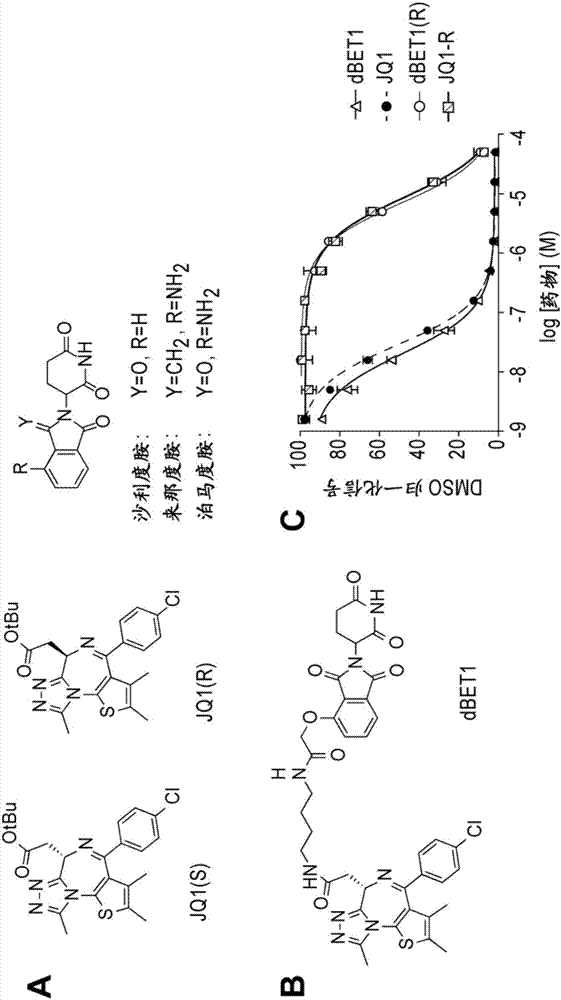
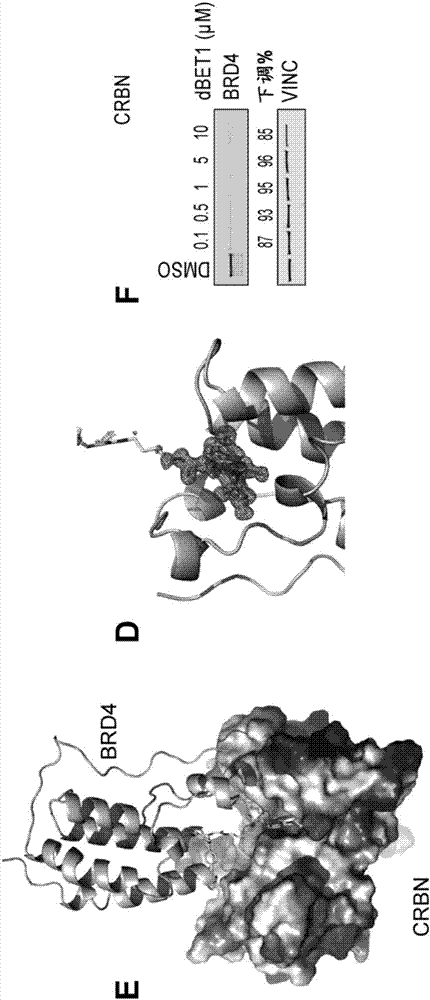
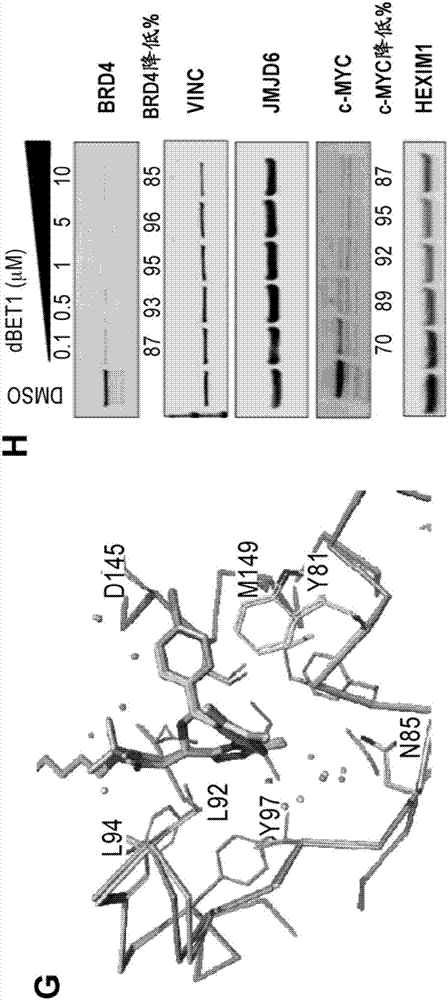
[1059] Example 1: Synthesis of dBET1
[1060]
[1061] (1) Synthesis of JQ-acid
[1062] JQ1 (1.0 g, 2.19 mmol, 1 equiv) was dissolved in formic acid (11 mL, 0.2M) at room temperature and stirred for 75 hours. The mixture was concentrated under reduced pressure to give a yellow solid (0.99 g, quantitative yield), which was used without purification. 1 H NMR (400MHz, methanol-d 4 )δ7.50–7.36(m,4H),4.59(t,J=7.1Hz,1H),3.51(d,J=7.1Hz,2H),2.70(s,3H),2.45(s,3H), 1.71 (s,3H). LCMS 401.33 (M+H).
[1063] Synthesis of N-(4-aminobutyl)-2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindoline- 4-yl)oxy)acetamide trifluoroacetate (Fischer et al. Nature 2014, 512, 49).
[1064] (2) Synthetic dBET1
[1065] Combine JQ-acid (11.3 mg, 0.0281 mmol, 1 equivalent) and N-(4-aminobutyl)-2-((2-(2,6-dioxopiperidin-3-yl)-1,3 -Dioxoisoindolin-4-yl)oxy)acetamide trifluoroacetate (14.5 mg, 0.0281 mmol, 1 equiv) was dissolved in DMF (0.28 mL, 0.1 M) at room temperature. Then DIPEA (14.7 μL, 0.0843...
Embodiment 2
[1066] Embodiment 2: Synthesis of dBET4
[1067]
[1068] N-(4-aminobutyl)-2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindoline-4- To a 0.1 M solution of ((R)oxy)acetamide trifluoroacetate salt in DMF (0.438 mL, 0.0438 mmol, 1.2 eq) was added (R)-JQ-acid (derived from ( R)-JQ1 prepared) (14.63 mg, 0.0365 mmol, 1 equiv). DIPEA (19.1 μL, 0.1095 mmol, 3 eq) and HATU (15.3 mg, 0.0402 mmol, 1.1 eq) were added and the mixture was stirred for 24 h, then diluted with MeOH and concentrated under reduced pressure. The crude material was purified by preparative HPLC to give a yellow solid (20.64 mg, 0.0263 mmol, 72%). 1 H NMR (400MHz, methanol-d 4 )δ7.79(dd, J=8.4,7.4Hz,1H),7.51(d,J=7.3Hz,1H),7.47–7.39(m,5H),5.11–5.06(m,1H),4.75(s ,2H),4.68(dd,J=8.8,5.5Hz,1H),3.47–3.31(m,5H),2.83–2.65(m,7H),2.44(s,3H),2.13–2.06(m,1H ),1.68(s,3H),1.67–1.60(m,4H). 13 C NMR (100MHz, cd 3 od)δ174.43,172.40,171.29,169.92,168.24,167.82,166.71,156.31,153.14,138.38,138.24,137.54,134.88,133.86,133.44,132....
Embodiment 3
[1069] Embodiment 3: synthetic dBET3
[1070]
[1071] N-(2-aminoethyl)-2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindoline-4- A 0.1 M solution of oxy)acetamide trifluoroacetate in DMF (0.475 mL, 0.0475 mmol, 1.2 equiv) was added to JQ-acid (15.86 mg, 0.0396 mmol, 1 equiv). DIPEA (20.7 μl, 0.1188 mmol, 3 equiv) and HATU (16.5 mg, 0.0435 mmol, 1.1 equiv) were then added and the mixture was stirred for 24 hours before purification by preparative HPLC to give a yellow solid (22.14 mg, 0.0292 mmol, 74%). 1 H NMR (400MHz, methanol-d 4 )δ7.82–7.75(m,1H),7.52–7.32(m,6H),5.04(dd,J=11.6,5.5Hz,1H),4.76(d,J=3.2Hz,2H),4.66(d ,J=6.6Hz,1H),3.58–3.35(m,6H),2.78–2.58(m,6H),2.48–2.41(m,3H),2.11–2.02(m,1H),1.70(d,J =11.8Hz,3H). 13 C NMR (100MHz, cd 3 od)δ174.38,171.26,171.19,170.26,168.86,168.21,167.76,166.72,156.27,153.14,138.44,138.36,138.19,134.87,133.71,132.31,131.57,131.51,129.90,129.86,121.81,119.36,117.95,69.48, 54.83, 50.52, 40.09, 39.76, 38.30, 32.09, 23.63, 14.40, 11.61. LC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com