Liposomal formulations, and methods of using and preparing thereof
A technology of liposomes, phospholipids, applied in the field of treatment of diseases and disorders, can solve problems such as unmet needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0363] The embodiments listed below represent some aspects of the disclosure.
[0364] 1. A composition comprising:
[0365] A liposome comprising:
[0366] i) 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE); and
[0367] ii) one or more stabilizers selected from the group consisting of:
[0368] (a) one or more phospholipids having a polar head group selected from the group consisting of glycerol, choline, phosphoric acid and serine and a fatty acid tail comprising C 10-28 aliphatic chain, and
[0369] (b) acid cholesteryl esters,
[0370] or both (a) and (b); and
[0371] iii) PEG conjugated to at least one phospholipid; and
[0372] at least one endogenous phosphorylated carbohydrate, wherein said at least one endogenous phosphorylated carbohydrate is encapsulated within said liposome.
[0373] 2. The composition of embodiment 1, wherein the liposomes further comprise cholesterol.
[0374] 3. The composition according to embodiment 2, wherein the cholesterol is...
Embodiment 1
[0601] Screening and selection of lipids for intracellular delivery of M1P
[0602] To select an appropriate lipid combination for intracellular delivery of M1P, the ability of different Lipo-M1P formulations to penetrate cell membranes, as well as facilitate endosomal escape and release of M1P active components into the cytosol was evaluated. GDP-mannose levels before and after Lipo-M1P treatment were measured using a functional cell-based assay as a measure of delivery and bioactivity of various liposome compositions. A total of 34 different Lipo-M1P formulations were prepared using a laboratory-scale thin-film hydration method. Cells were treated with different Lipo-M1P formulations up to a total lipid concentration of 5.4 mM. Cell viability was determined using the XTT cell proliferation assay (ThermoFisher Scientific) and 28 of the 33 tested formulations maintained cell viability above 80% of the required level. After treatment, cells were harvested, GDP-mannose was ext...
Embodiment 2
[0610] Stability study of initial formulation of Lipo-M1P
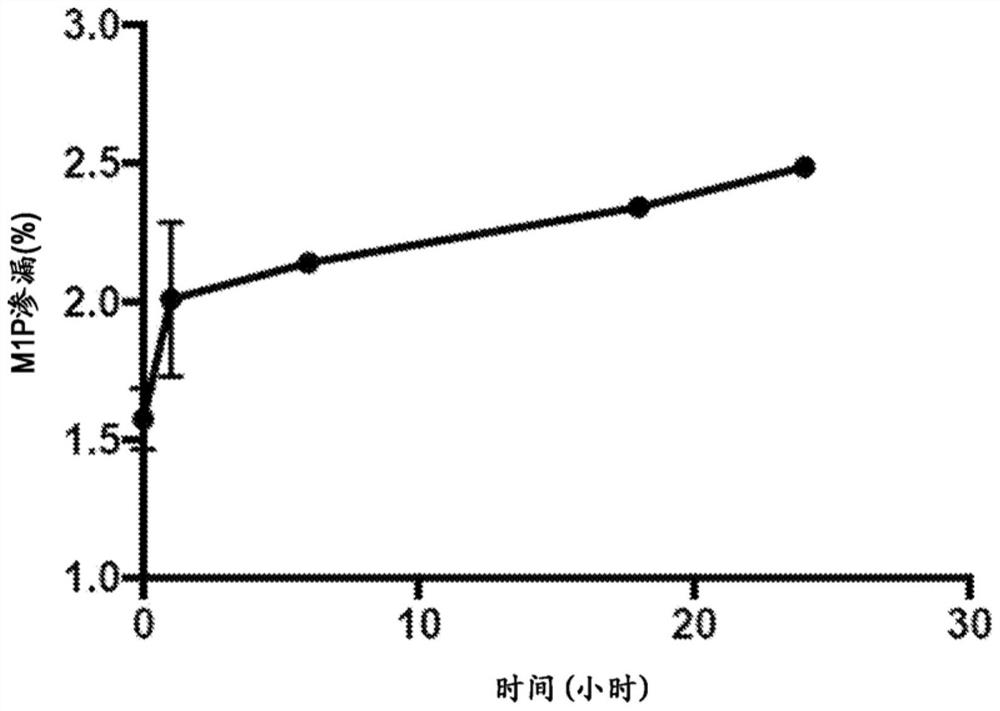
[0611] In vitro assessment of M1P leakage by liposomes was performed to characterize the physical state of the lipid bilayer and encapsulated M1P to understand M1P stability inside the liposomes under physiological conditions. After washing freshly prepared Lipo-M1P particles to remove free unencapsulated M1P, Lipo-M1P was resuspended in PBS pH 7.4 and stored at 37 °C. The liposome composition is DOPE:DOPC:DSPE-PEG2000=48.5:48.5:3 (mol%). At several time points up to 24 hours, M1P that had leaked from the particles was separated using a 10 kDa molecular weight cut-off membrane (MWCO) centrifugal filter, and the liposomes in the filter were brought to reach original volume. The filtrate containing leaked M1P was quantified by bicinchoninic acid assay (BCA assay). M1P leakage was calculated as a percentage of total Lipo-M1P and plotted over time as figure 1 shown. Negligible leakage was observed, with only 2.5% re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com