Electrochemical recovery method of heavy rare earth metals
A recycling method and heavy rare earth technology, which can be used in optics, process efficiency improvement, instruments, etc., and can solve problems such as recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0056] Example: Three-electrode electrolytic reduction (electrolytic reduction) system
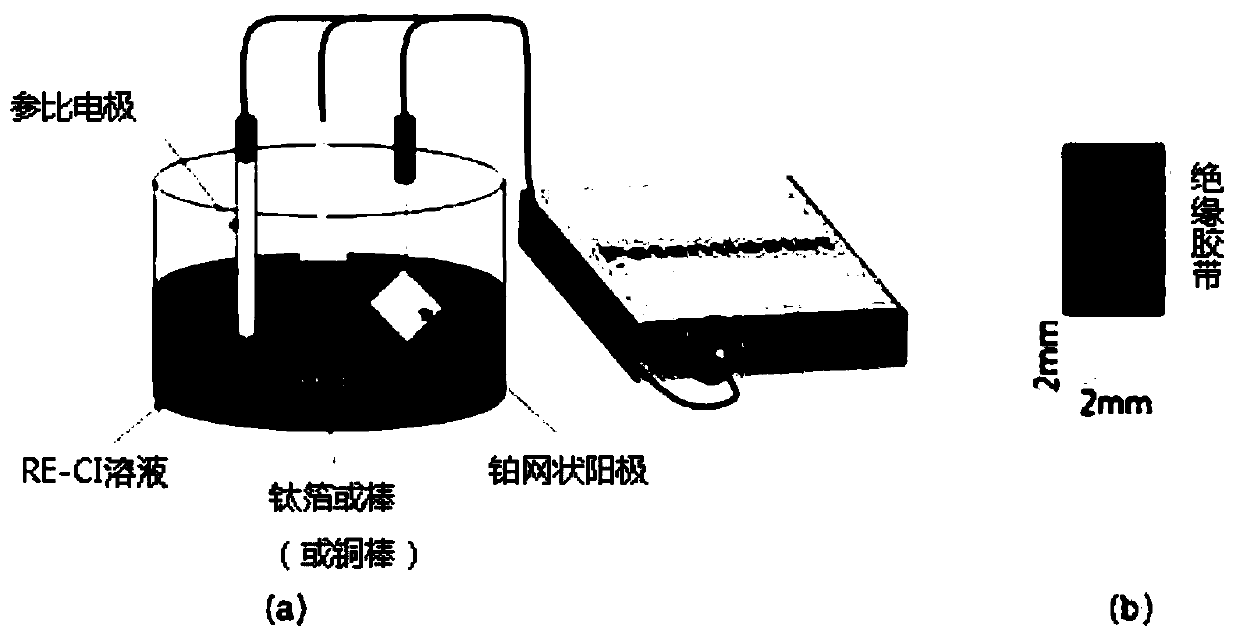
[0057] The three-electrode electrolytic reduction system used in the present invention includes a potentiostat (Potentiostat), a working electrode, a counter electrode, a reference (Reference) electrode and RE-Cl solution (Rare Earth (Rare Earth)-chloride solution) as a sample . figure 1 is a schematic diagram of a three-electrode electrolytic reduction system, figure 1 Part (a) is a schematic diagram of a three-electrode electrolytic reduction system using titanium foil as a working electrode, figure 1 Part (b) is a schematic diagram of the working electrode. The potentiostat used in the present invention is Iviumstat and Gamry equipment, in three electrodes, has used titanium rod (Titanium rod, (r=5mm, l=2mm)) or titanium foil (2mm * 2mm) as working electrode, As the counter electrode, a platinum mesh electrode (Platinum meshelectrode) was used, and as the reference electrode, an accu...
experiment example 1
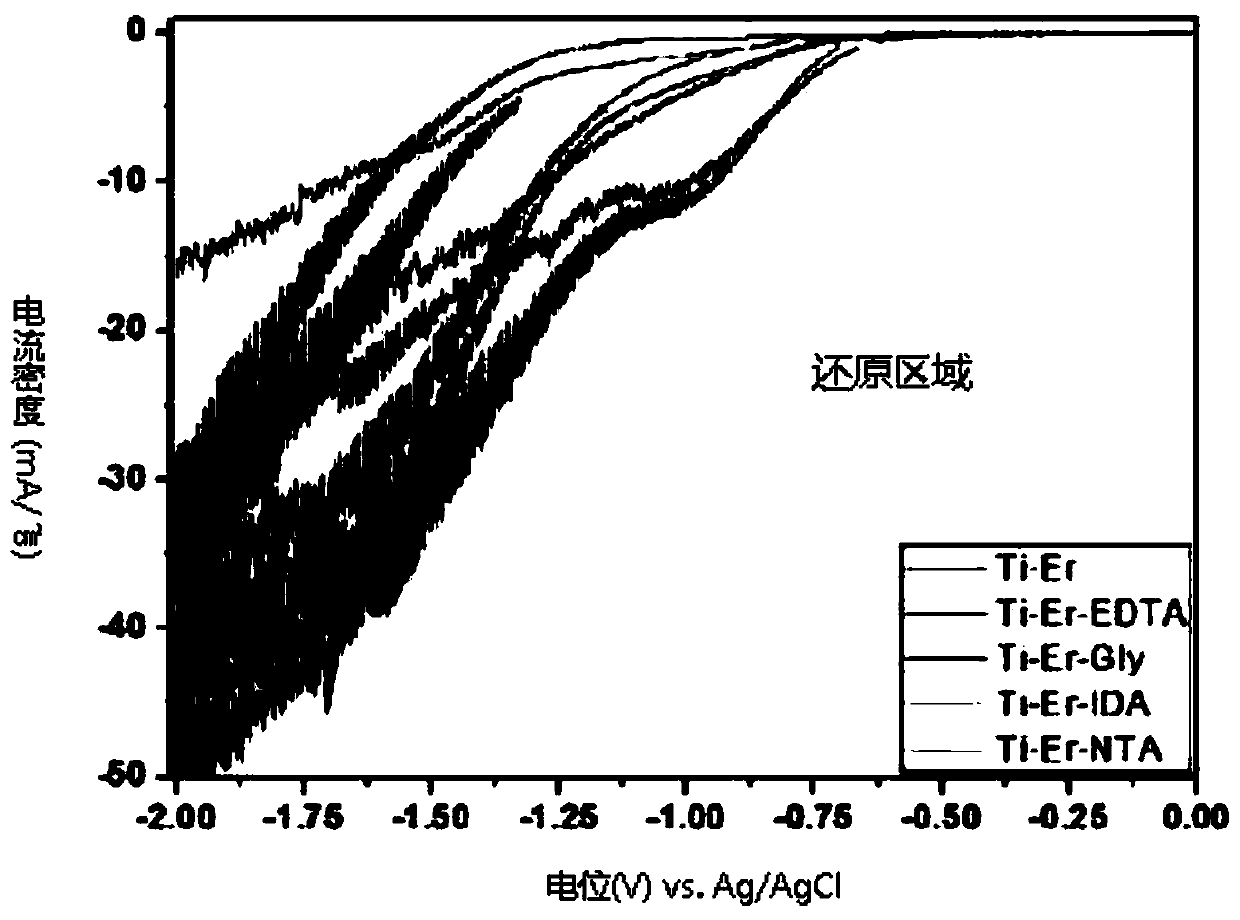
[0061]Experimental example 1: Cyclic voltage and current experiment based on complexing agents
[0062] The complexing agent is an organic compound combined with metal ions in a circular arrangement structure. The complexing agent to be used in the three-electrode electrolytic reduction system ensured in Example 1 is glycine, ethylenediaminetetraacetic acid, imino Diacetic acid and nitrilotriacetic acid. And 2M of the above-mentioned complexing agent was used. The pH of the basic solution (a mixed solution of hydrochloric acid and distilled water) was fixed at 1, and RECl was added to the above basic solution 3 .6H 2 O powder to prepare RE-Cl solution. As the heavy rare earth metal ions in the RE-Cl solution, erbium, thulium, and ytterbium were used, and the concentrations of the respective metal ions were set to 1M. The titanium rod was used as the working electrode, and as the control group, the experiment was carried out under the same conditions except that no complexi...
experiment example 2
[0071] Experimental example 2: Cyclic voltage and current experiment based on pH conditions
[0072] Except that KOH is used to adjust the pH of the RE-Cl solution and the heavy rare earth metal ions of the RE-Cl solution are fixed as erbium, all other conditions are the same as those of the complexing agent-based cyclic voltage-current experiment in Experimental Example 1. The RE-Cl solution used in the present invention is the simulated solution of mineral waste water, thereby although initial waste water has acidity, but considering carrying out neutralization treatment, the reduction situation of the erbium when pH is 1 and 7 has been observed, and for For comparison, a reduction experiment of erbium at a pH of 12 was carried out.
[0073] Figure 6a , Figure 6b and Figure 6c Respectively represent the current density based on the complexing agent measured by cyclic voltammetry when the heavy rare earth metal ion is erbium when the pH is 1, 7 and 12, respectively.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com