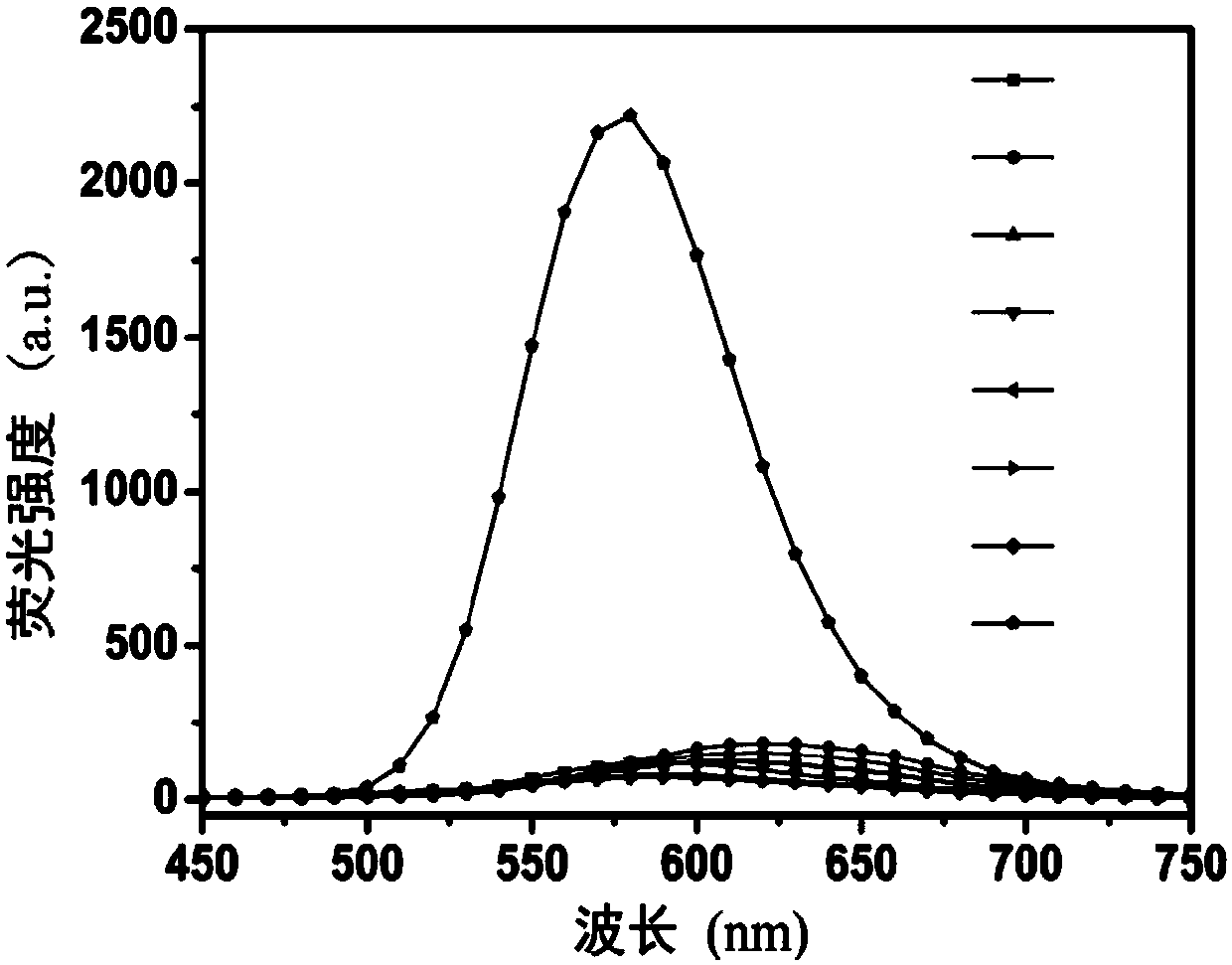
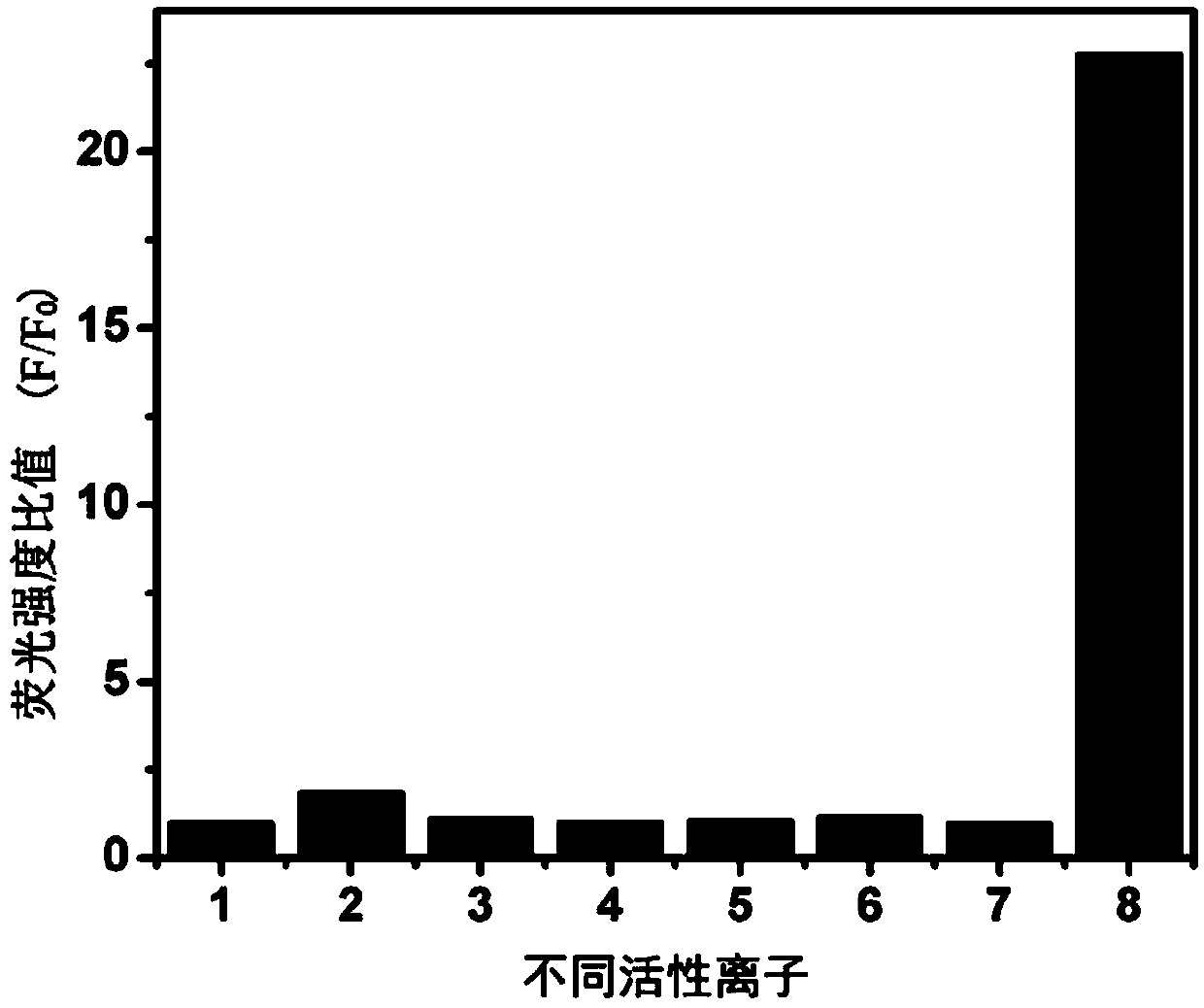
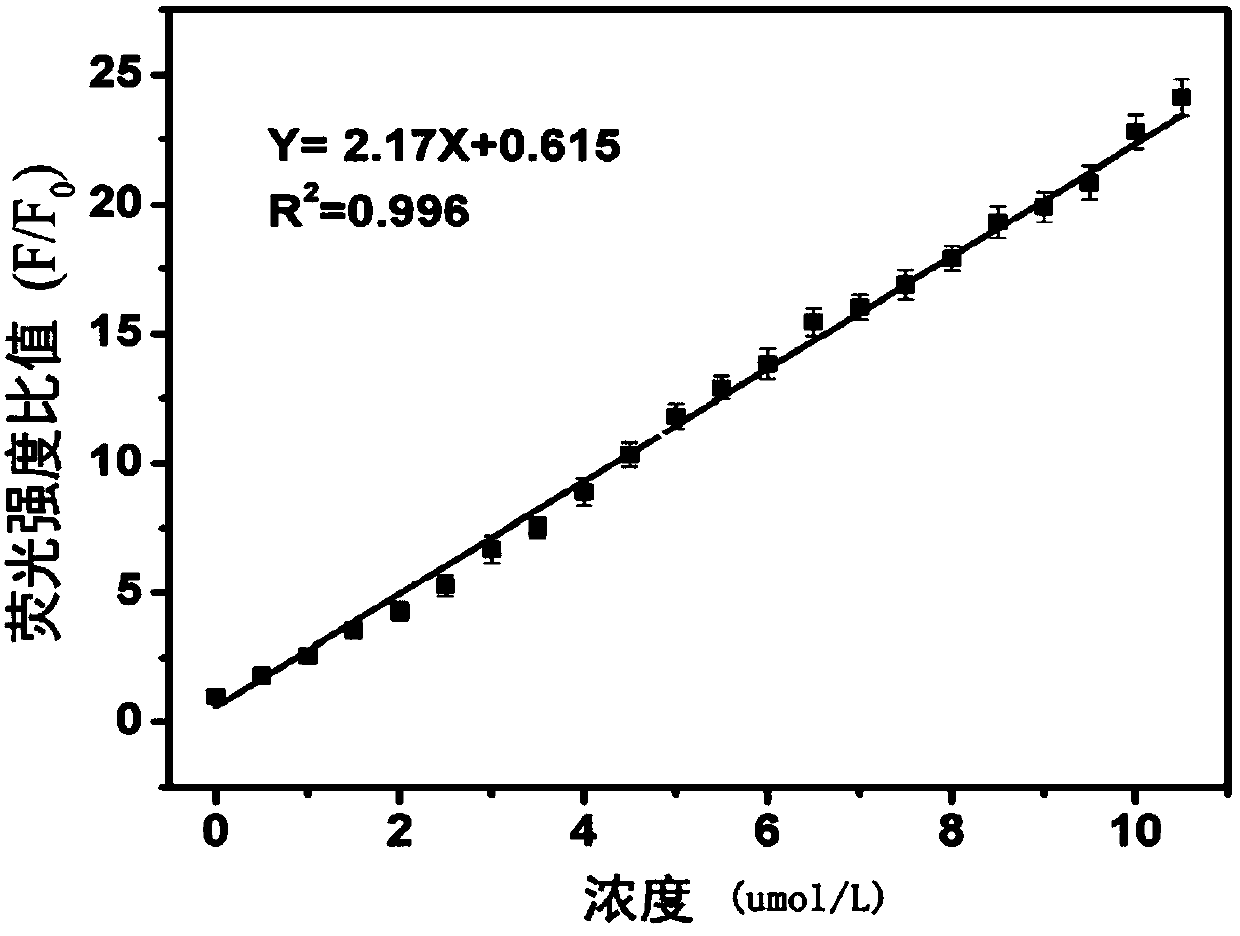
A fluorescent probe for detecting intracellular peroxynitrite ion and its synthesis method
A peroxynitrite and fluorescent probe technology, applied in the field of chemistry, can solve the problems of compound synthesis, long reaction time, and short emission wavelength, and achieve the effects of simple structure, short response time, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthetic route is as follows (formula 2):
[0042]
[0043] 1) Synthesis of 6-dimethylamino-1-tetralone
[0044] Dissolve 6-amino-1,2,3,4-tetrahydro-1-naphthone (325mg, 2mmol) and potassium carbonate (830mg, 6mmol) in 5mL of N,N-dimethylformamide solution, and then Add iodomethane (430 mg, 3 mmol), then heat to 45 ° C, and stir for 24 h to obtain the first mixed solution; after the reaction is completed, the first mixed solution is cooled to room temperature; 10 mL of the first mixed solution is added to the first mixed solution Deionized water, extracted three times with ethyl acetate, and washed several times with a large amount of water and saline, the organic layer after extraction was dried with anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and then column chromatography was carried out (with a volume ratio of 6 : 1 mixed solution of petroleum ether and ethyl acetate is eluent), and the white solid obtained is 6-dimethylamino-...
Embodiment 2
[0052] The synthetic route is as shown in (formula 3):
[0053]
[0054] 1) Synthesis of 6-dimethylamino-1-tetralone
[0055] Dissolve 6-amino-1,2,3,4-tetrahydro-1-naphthone (325mg, 2mmol) and potassium carbonate (830mg, 6mmol) in 5mL of N,N-dimethylformamide solution, and then Add 430 mg iodomethane (3 mmol), then heat to 45° C., stir for 24 h to obtain the first mixed liquid. After the reaction was completed, the first mixed solution was cooled to room temperature, 10 mL of deionized water was added to the first mixed solution, extracted three times with ethyl acetate, and washed several times with a large amount of water and saline. The extracted organic phase was dried with anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation, and then separated by column chromatography (using a mixture of petroleum ether and ethyl acetate with a volume ratio of 6:1 as the eluent) to obtain a white solid It is 6-dimethylamino-1-tetralone, the yield is 160 mg,...
Embodiment 3
[0063] The synthetic route is as follows (formula 4):
[0064]
[0065] 1) Synthesis of 6-dicyclopropylamino-1-tetralone
[0066] Dissolve 6-amino-1,2,3,4-tetrahydro-1-naphthalenone (325 mg, 2 mmol) and potassium hydroxide (336 mg, 6 mmol) in 5 mL of DMSO solution, then add cyclopropane bromide (363mg, 3mmol), heated to 60°C and stirred for 24h to obtain the first mixed solution; after the reaction was completed, the first mixed solution was cooled to room temperature, and 20 mL of deionized water was added to the first mixed solution , extracted three times with ethyl acetate, and washed several times with a large amount of water and saline, the organic phase after the extraction was dried with anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation, and then separated by column chromatography (with a volume ratio of 8:1 The mixed solution of petroleum ether and ethyl acetate is the eluent), and the white solid obtained is 6-dicyclopropylamino-1-tet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com