Ferrocyanogen positive material, preparation method and application thereof
A positive electrode material, ferrocyanide-based technology, applied in ferricyanide, battery electrodes, metal cyanide, etc., can solve problems such as unsatisfactory cycle stability, poor crystallinity of ferrocyanide, and low capacity of sodium-ion batteries. Achieve the effect of good crystallinity, short cycle time and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
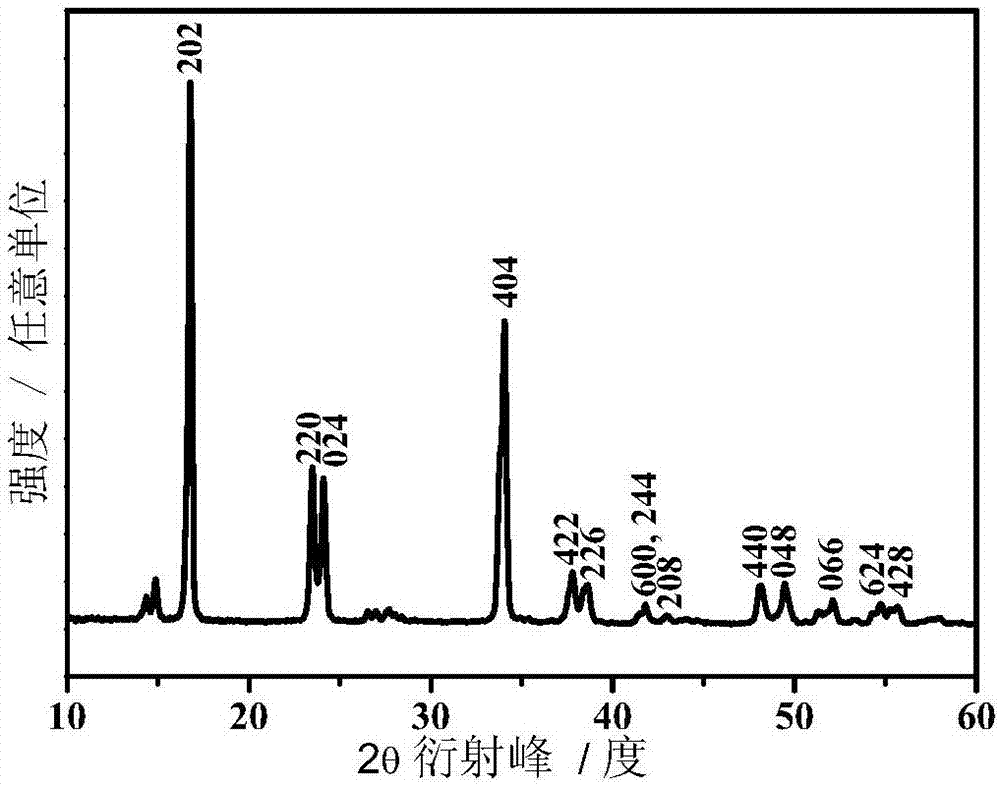
[0034] Sodium ferrocyanide is dissolved in deionized water, and stirred evenly to obtain solution A whose concentration is 0.1mol / L in terms of ferrocyanide ion; manganous chloride is dissolved in deionized water, and stirred evenly to obtain 2+ Solution B whose meter concentration is 0.2mol / L, wherein the molar weight of manganous chloride is 2.5 times of sodium ferrocyanide, then sodium hydroxide is added in the solution B and stirred (sodium hydroxide and manganous chloride The molar ratio is 2.5:1), to get Mn(OH) 2 Suspension C: Mix solution A and suspension C, add acetic acid, the molar amount of acetic acid is 5 times that of manganese chloride, undergo a hydrothermal reaction at 90°C for 8 hours, then cool, wash and dry to obtain a ferrocyanide positive electrode Material. Through ICP analysis, the x value of the product is 1.7, the y value is 0.8, the lattice structure is rhombohedral phase, and the particle size is 200-400 nm.
[0035] figure 1 For the X-ray diffra...
Embodiment 2
[0047] Dissolve sodium ferrocyanide in deionized water and stir evenly to obtain solution A with a concentration of 0.2 mol / L in terms of ferrocyanide ion; dissolve manganous sulfate and ferrous chloride with a molar ratio of 1:1 in In deionized water, stir evenly to obtain Mn 2+ and Fe 2+ A total concentration of 0.4mol / L solution B (wherein the total molar weight of manganese sulfate and ferrous chloride is 3 times that of sodium ferrocyanide), then sodium hydroxide is added to solution B and stirred (hydroxide The total molar ratio of sodium to manganous sulfate and ferrous chloride is 3:1), to obtain Mn 0.5 Fe 0.5 (OH) 2 Suspension C: Mix solution A and suspension C, add tartaric acid, the molar amount of tartaric acid is 4 times that of manganese chloride, undergo hydrothermal reaction at 80°C for 10 hours, then cool, wash and dry to obtain a ferrocyanide positive electrode material, the chemical formula is Na x M[Fe(CN) 6 ] y , where, x=1.65, y=0.75; the lattice s...
Embodiment 3
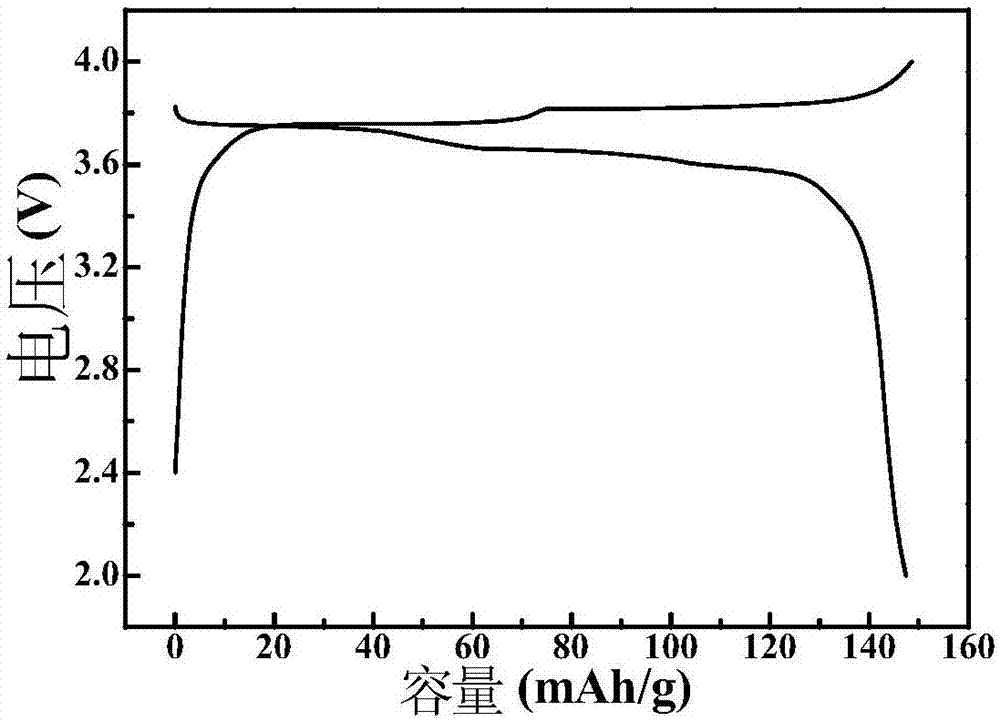
[0050] Sodium ferrocyanide is dissolved in deionized water, and stirred evenly to obtain solution A whose concentration is 0.3mol / L in terms of ferrocyanide ion; ferrous chloride is dissolved in deionized water, stirred evenly to obtain 2+ The solution B whose meter concentration is 0.65mol / L, wherein the molar weight of ferrous chloride is 2.5 times of sodium ferrocyanide, then sodium hydroxide is added in the solution B and stirred (sodium hydroxide and ferrous chloride The molar ratio is 2.5:1), to get Fe(OH) 2 Suspension C: Mix solution A and suspension C, add propionic acid, the molar weight of propionic acid is 3 times that of ferrous chloride, undergo hydrothermal reaction at 90°C for 10 hours, then cool, wash and dry to obtain ferrocyanide Based cathode material, the chemical formula is Na x M[Fe(CN) 6 ] y , where, x=1.62, y=0.76; the lattice structure is rhombohedral phase, and the particle size is 200-400nm.
[0051] The ferrocyanogen-based material prepared in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com