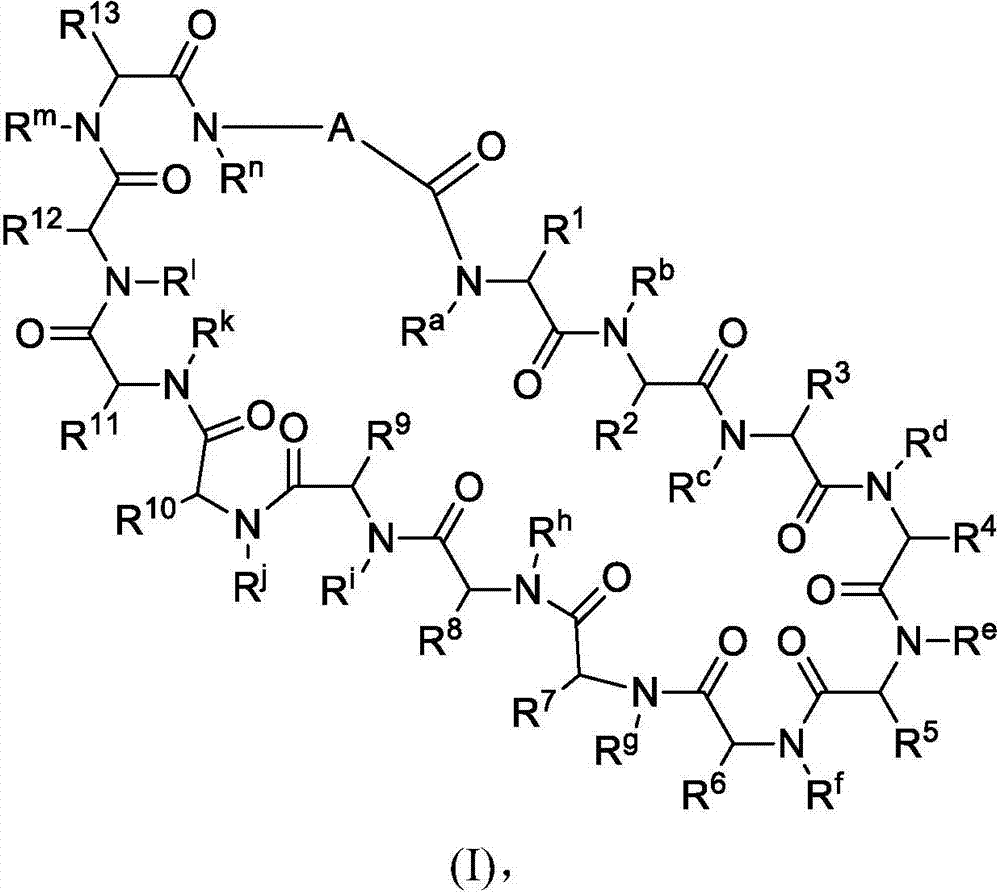
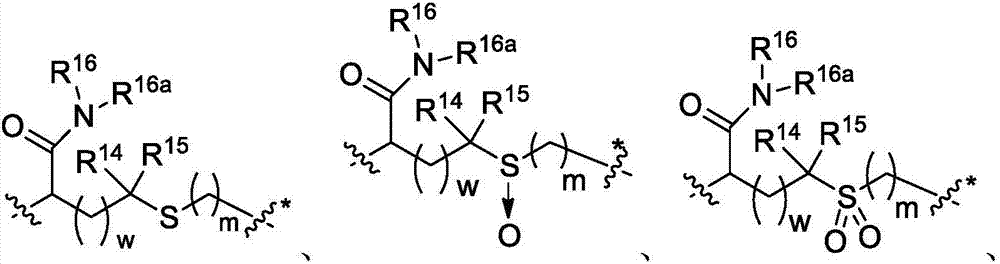
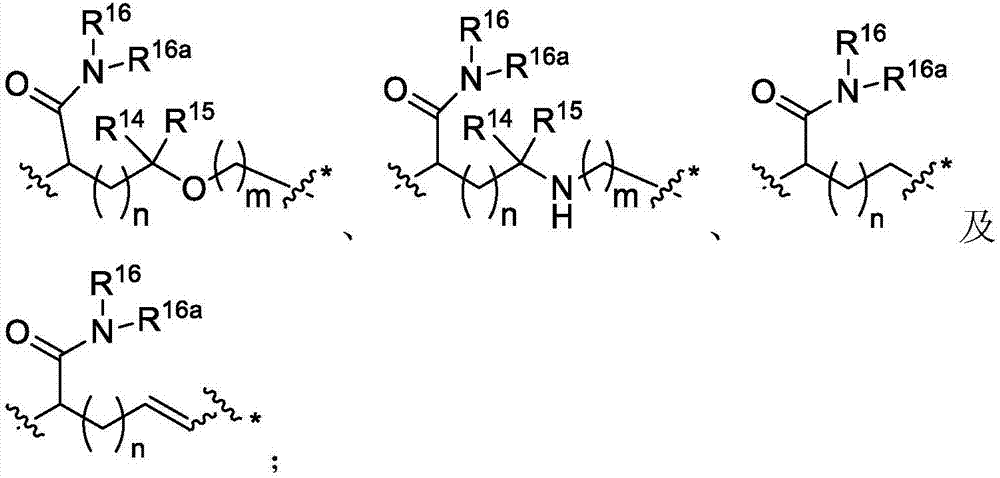
Immunomodulators
A technology selected from, methyl, applied in the direction of antiviral agents, allergic diseases, extracellular fluid diseases, etc., can solve the lack of MYPPY motifs and other problems, and achieve the effect of enhancing the functional activity of T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0349] Preparation of INT-1300Z
[0350]
[0351] The following peptides were synthesized according to the above procedure on a 0.4 mmol scale. Underlined steps employed a double coupling procedure, and a 30 min single coupling was used to couple residues in italics. ClAc- Tyr -[N-Me]Ala- Asn -Pro-Dap- Leu -Hyp-Trp-Dab-[(S)-2-Amino-3-(1-(carboxymethyl)-1H-indol-3-yl)propionic acid]-[ N-Me] Nle -[N-Me]Nle-Leu-Cys-Gly; wherein Gly is added to 2-chlorotrityl resin. Cleavage from the resin was achieved by shaking the resin in 20% hexafluoroisopropanol / DCM for 5 min followed by filtration. The filtrate was concentrated in vacuo to provide the desired product. HPLCRT=2.21min, column: Waters Aquity UPLC BEH C18 2.1×50mm 1.7-μm particles; mobile phase A: acetonitrile containing 0.05% TFA; mobile phase B: water containing 0.05% TFA; gradient: within 1.5 minutes 2- 98% B, then hold at 98% B for 1.55 minutes; flow rate: 0.8 mL / min.
[0352] Preparation of INT-130AA
[0353...
Embodiment 13080
[0363] Preparation of Example 13080
[0364]
[0365] Step 1: To a solution of intermediate 1300Z (61.8 mg, 0.021 mmol) in DMF (537 μl) was added HATU (12.25 mg, 0.032 mmol) followed by Huenig's base (11.25 μl, 0.064 mmol). Finally, benzylamine (3.52 μl, 0.032 mmol) was added, and the resulting solution was stirred at room temperature. Within 3h, product had formed as shown by LC / MS. Water was added, and the resulting mixture was filtered to afford a solid 3-((7R,10S,13S,16S,19S,22S,25S)-25-((2S,4R)-4-(tert-butoxy)-1-( (S)-2-((S)-2-((S)-1-((S)-2-((S)-2-((S)-3-(4-(tert-butoxy) Phenyl)-2-(2-chloroacetylamino)-N-methylpropionylamino)propionylamino)-4-oxo-4-(tritylamino)butyryl)pyrrolidine-2-methane Amino)-3-((tert-butoxycarbonyl)amino)propionylamino)-4-methylpentanoyl)pyrrolidine-2-carboxamido)-19-((1-(2-(tert-butyl Oxy)-2-oxoethyl)-1H-indol-3-yl)methyl)-22-(2-((tert-butoxycarbonyl)amino)ethyl)-13,16-dibutyl Base-10-isobutyl-14,17-dimethyl-3,6,9,12,15,18,21,24-octaoxo-1-p...
Embodiment 13081
[0370] Preparation of Example 13081
[0371]
[0372] 2-((6S,9S,12S,18R,21S,24S,27S,30S,33S,36S,38aS,40R,44S,47S,49aS)-30,36-bis(( 1H-indol-3-yl)methyl)-6-(2-amino-2-oxoethyl)-44-(3-amino-3-oxopropyl)-24,27-dibutyl -40-Hydroxy-12-(4-hydroxybenzyl)-33,47-bis(hydroxymethyl)-21-isobutyl-9,10,25,28-tetramethyl-5,8,11, 14, 20, 23, 26, 29, 32, 35, 38, 43, 46, 49-Tetradecyloxotetraoctahydrodipyrrolo[2,1-g 1 :2',1'-x][1,4,7,10,13,16,19,22,25,28,31,34,37,40,43]thiatetradetraazepine To a solution of alkane-18-carboxamido)acetic acid (21.2mg, 0.012mmol) was added HATU (5.72mg, 0.015mmol) followed by Huenig base (8.08μl, 0.046mmol) in DMF and 5- Mixture of azidopentan-1-amine (14.82 mg, 0.116 mmol). The mixture was stirred at room temperature. After about 1 h, a small amount of product was seen and most of the SM remained. After another hour, the LC / MS did not change much. Additional HATU and amine mixture were added sequentially, and the yellow solution was stirred at room temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com