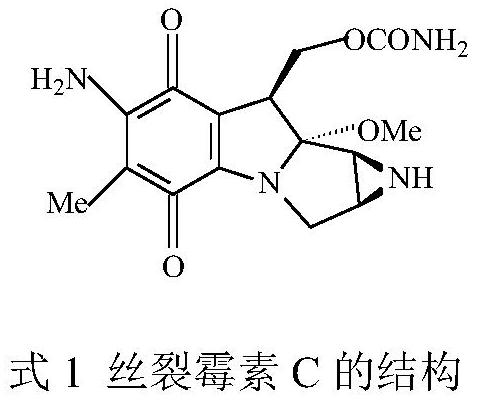
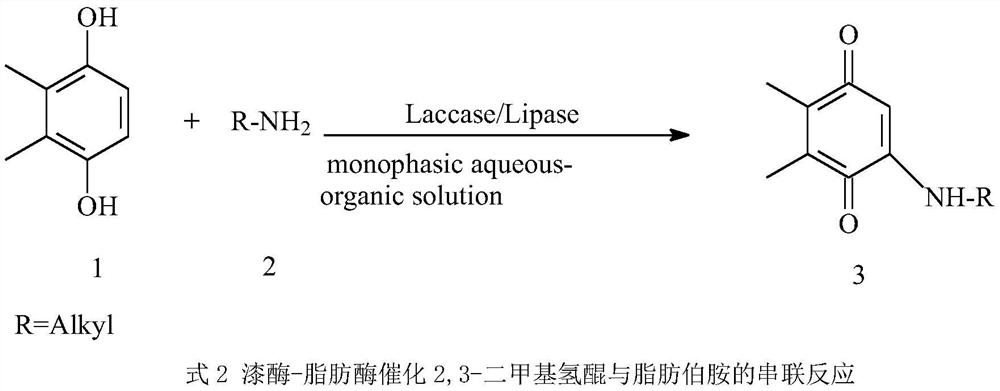
A method for synthesizing 2,3-dimethyl-5-alkylamino-1,4-benzoquinone in one pot with two enzymes
A technology of dimethyl hydroquinone and dimethyl, which is applied in the field of double-enzyme one-pot synthesis of 2,3-dimethyl-5-alkylamino-1,4-benzoquinone, can solve the problem of affecting C-N cross-coupling reaction , low efficiency of aminoquinone compounds, low product yield and other problems, to solve the problem of inhibition of benzoquinone products, to avoid the effect of relatively expensive and easy-to-operate reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A method for synthesizing 2,3-dimethyl-5-alkylamino-1,4-benzoquinone in one pot with two enzymes, the specific steps are as follows: (1) Preparation of Myceliophthora thermophila laccase / Aspergillus niger lipase Double-enzyme cross-linking enzyme aggregates, the process is as follows: 1.0Kg Myceliophthora thermophila laccase enzyme powder (≥800U / g) and Aspergillus niger lipase enzyme powder (≥800U / g) mixture (ratio of enzyme activity 1:10), dissolved in 6L of pH 7.0 phosphate buffer solution, added ammonium sulfate under slow stirring at 150rpm at 4°C to make the concentration 0.3kg / L, continued to stir for 30min, and added the cross-linking agent glutaraldehyde Make the concentration 0.2%, cross-link for 2 hours, centrifuge the mixed solution at 4°C, 5000×g for 10 minutes, remove the supernatant, wash the precipitate with deionized water, and freeze-dry to obtain Myceliophthora thermophila laccase / Aspergillus niger The enzyme lipase double enzyme cross-links enzyme agg...
Embodiment 2
[0036] A method for synthesizing 2,3-dimethyl-5-alkylamino-1,4-benzoquinone in one pot with two enzymes, the specific steps are as follows: (1) Preparation of Myceliophthora thermophila laccase / Aspergillus niger lipase Double-enzyme cross-linking enzyme aggregates, the process is as follows: 0.5Kg Myceliophthora thermophila laccase enzyme powder (≥800U / g) and Aspergillus niger lipase enzyme powder (≥800U / g) mixture (ratio of enzyme activity 1:9), dissolved in 3L of pH 7.0 phosphate buffer, added ammonium sulfate under slow stirring at 150rpm at 4°C to make the concentration 0.5kg / L, continued to stir for 30min, and added the cross-linking agent glutaraldehyde Make the concentration 0.3%, cross-link for 2 hours, centrifuge the mixed solution at 4°C, 5000×g for 10 minutes, remove the supernatant, wash the precipitate with deionized water, and freeze-dry to obtain Myceliophthora thermophila laccase / Aspergillus niger The enzyme lipase double enzyme cross-links enzyme aggregates. ...
Embodiment 3
[0039] A method for synthesizing 2,3-dimethyl-5-alkylamino-1,4-benzoquinone in one pot with two enzymes, the specific steps are as follows: (1) Preparation of Myceliophthora thermophila laccase / Aspergillus niger lipase Double-enzyme cross-linking enzyme aggregates, the process is as follows: 3.0Kg Myceliophthora thermophila laccase enzyme powder (≥800U / g) and Aspergillus niger lipase enzyme powder (≥800U / g) mixture (ratio of enzyme activity 1:5), dissolved in 20L of pH 7.0 phosphate buffer solution, added ammonium sulfate under slow stirring at 150rpm at 4°C to make the concentration 0.4kg / L, continued stirring for 30min, and added the cross-linking agent glutaraldehyde Make the concentration 0.1%, cross-link for 1 hour, centrifuge the mixed solution at 4°C, 5000×g for 10 minutes, remove the supernatant, wash the precipitate with deionized water, and freeze-dry to obtain Myceliophthora thermophila laccase / Aspergillus niger The enzyme lipase double enzyme cross-links enzyme agg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com