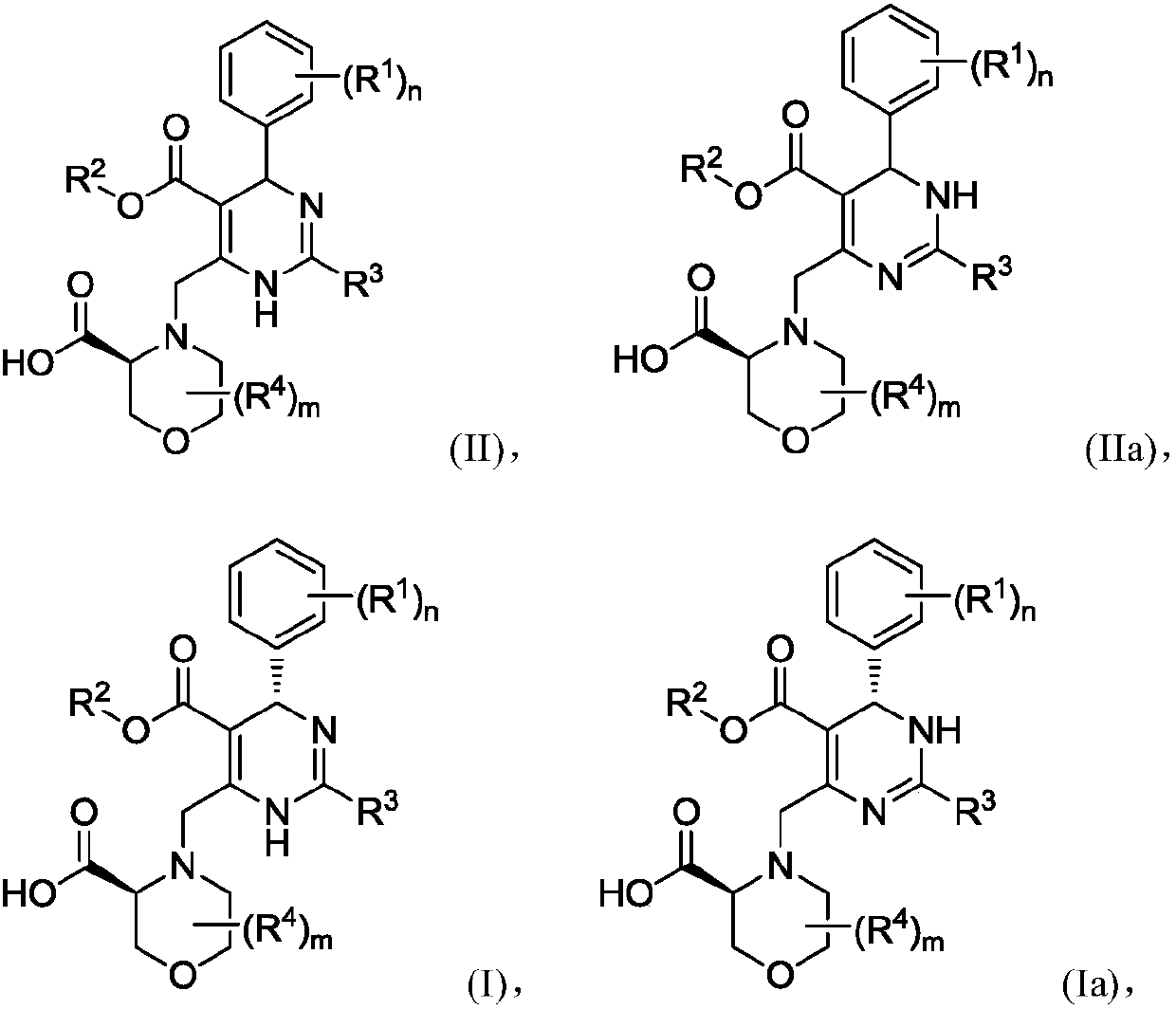
Preparation method of dihydropyrimidine derivatives and their acid adducts
An acid addition and compound technology, which is applied in the preparation of acid adducts, optically pure dihydropyrimidine derivatives or the preparation of tautomers thereof, can solve the problem of inability to use large-scale production and high requirements for experimental instruments. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
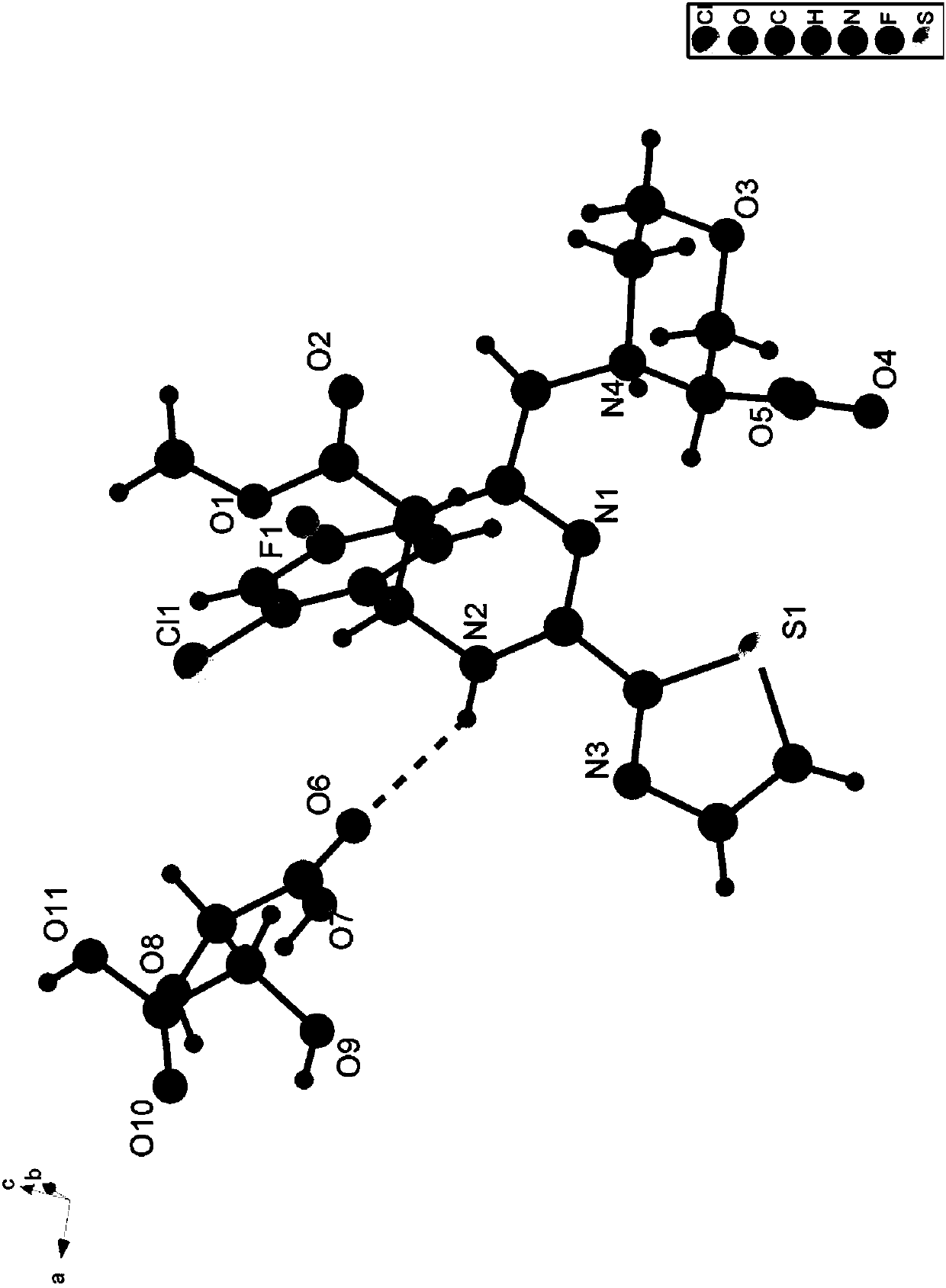
[0199] Example 1: (S)-4-(((R)-6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl)-3, Preparation of 6-dihydropyrimidin-4-yl)methyl)morpholine-3-carboxylic acid
[0200]
[0201] Add (3S)-4-((6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl)-3,6 -Dihydropyrimidin-4-yl)methyl)morpholine-3-carboxylic acid (1g) and ethanol (5mL). The mixture was stirred at room temperature for 4 hours and filtered. The filtrate was concentrated under reduced pressure and the solvent was removed to obtain (S)- 4-(((R)-6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl)-3,6-dihydropyrimidine-4- ((Yl)methyl)morpholine-3-carboxylic acid is a yellow foamy solid (0.46 g, 92%) with a chiral purity of 95%.
[0202] MS(ESI,pos.ion)m / z:494.9[M+H] + ;
[0203] 1 H NMR(400MHz, DMSO-d 6 ): δ12.52(br,1H), 9.86(s,1H), 8.03(d,1H), 7.94(d,1H), 7.43-7.38(m,2H), 7.16(td,1H), 6.04( s, 1H), 4.24 (d, 1H), 4.06-3.97 (m, 2H), 3.84 (dd, 1H), 3.73-3.66 (m, 2H), 3.64-3.59 (m, 1H), 3.51 ...
Embodiment 2
[0209] Example 2: (S)-4-(((R)-6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl)-1, Preparation of L-tartaric acid complex (c) of 6-dihydropyrimidin-4-yl)methyl)morpholine-3-carboxylic acid (method one)
[0210]
[0211] Add (3S)-4-((6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl)-3,6 to a 100mL three-necked flask in sequence -Dihydropyrimidin-4-yl)methyl)morpholine-3-carboxylic acid (7g, 14.14mmol), ethanol (45mL) and L-tartaric acid (1.2g, 8mmol), the resulting mixture was heated to 55°C, kept and stirred for 30min . Then the heating was turned off, and after the temperature was lowered to 25°C, the temperature was kept and stirred for 16 hours. After the reaction, filter, wash the filter cake with ethanol (10 mL), and then vacuum dry at 65°C for 8 hours to obtain (S)-4-(((R)-6-(2-chloro-4-fluorophenyl)- The L-tartaric acid complex of 5-(methoxycarbonyl)-2-(thiazol-2-yl)-1,6-dihydropyrimidin-4-yl)methyl)morpholine-3-carboxylic acid is a yellow ...
Embodiment 3
[0229] Example 3: (S)-4-(((R)-6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl)-1, Preparation of L-tartaric acid complex (c) of 6-dihydropyrimidin-4-yl)methyl)morpholine-3-carboxylic acid (method 2)
[0230]
[0231] Add (3S)-4-((6-(2-chloro-4-fluorophenyl)-5-(methoxycarbonyl)-2-(thiazol-2-yl)-3,6 to a 100mL three-necked flask in sequence -Dihydropyrimidin-4-yl)methyl)morpholine-3-carboxylic acid (7g, 14.14mmol) and ethanol (45mL), the mixture was stirred at room temperature for 4 hours and filtered. The obtained filtrate was transferred to a single-neck flask (100 mL), and then the temperature was raised to 55° C., and L-tartaric acid (1.2 g, 8 mmol) was added, and the resulting mixture was kept warm and stirred for 30 minutes. Subsequently, the heating was turned off and the temperature was lowered (7°C / hour). After the temperature was lowered to 25°C, the temperature was kept and stirred for 16 hours. Filter, wash with ethanol (10 mL), and then vacuum dry at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com