Lna-based mutant enrichment next-generation sequencing assays
A DNA molecule and sequence technology, applied in the field of LNA-based mutation enrichment next-generation sequencing assay, which can solve the problem of complex tumor-specific mutations and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0057] The invention is further illustrated in the following examples, which in no way limit the scope of the invention described in the claims.
[0058] method
[0059] The following method is used in the example shown below.
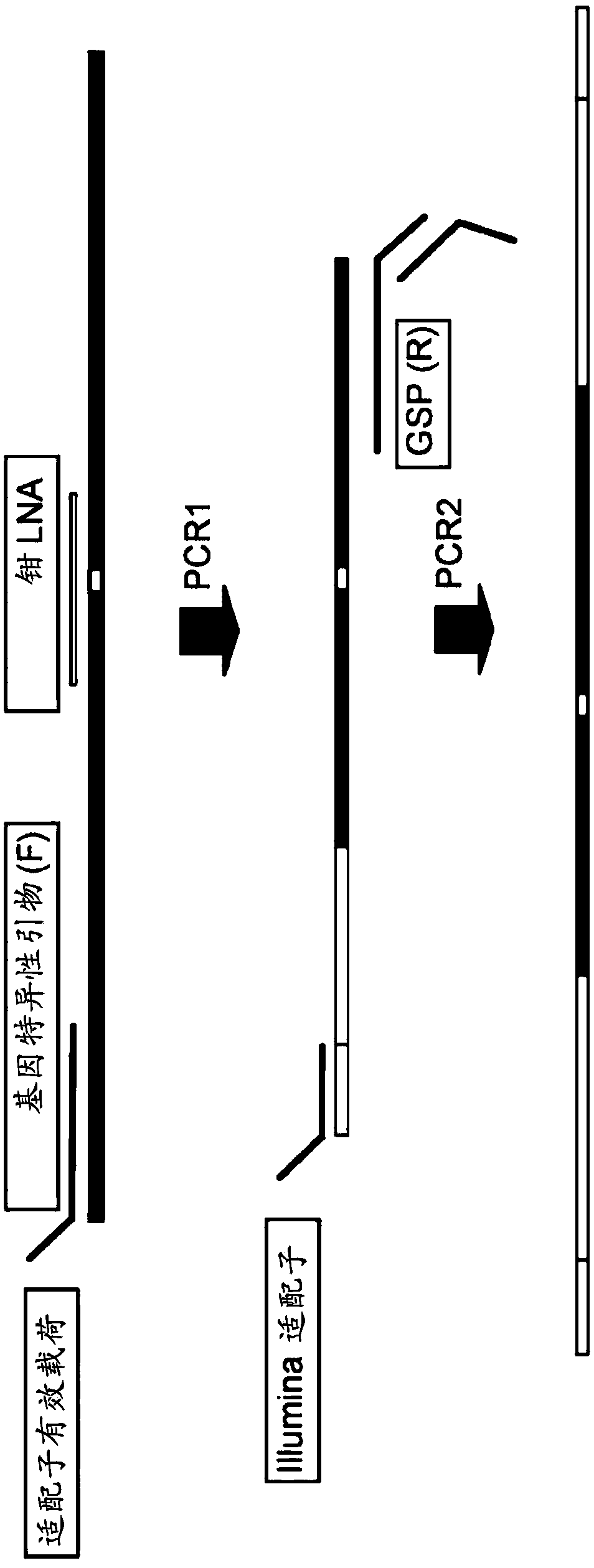
[0060] Genomic DNA (gDNA) from circulating tumor cells (CTCs) or circulating tumor DNA (ctDNA) was extracted using the Qiagen AllPrep DNA / RNA Micro Kit or Qiagen Circulating Nucleic Acid Kit, respectively, according to the manufacturer's protocol. Semifunctional sequencing libraries were prepared by combining DNA templates with new semifunctional gene-specific primers, matching gene-specific LNA clamps with the KAPA HiFi Hot-Start PCR Kit (KAPA Biosystems), and performing 25 rounds of primer extension, primer extension represents a critical step in mutant enrichment.
[0061] Next, 0.4X Solid Phase Reversible Immobilization (SPRI) bead cleanup was performed with Agencourt Ampure XP beads (Beckman Coulter, USA) according to the manufacturer's protocol...
example 1
[0078] Example 1. ESR1 and PIK3CA mutation analysis using enrichment sequencing
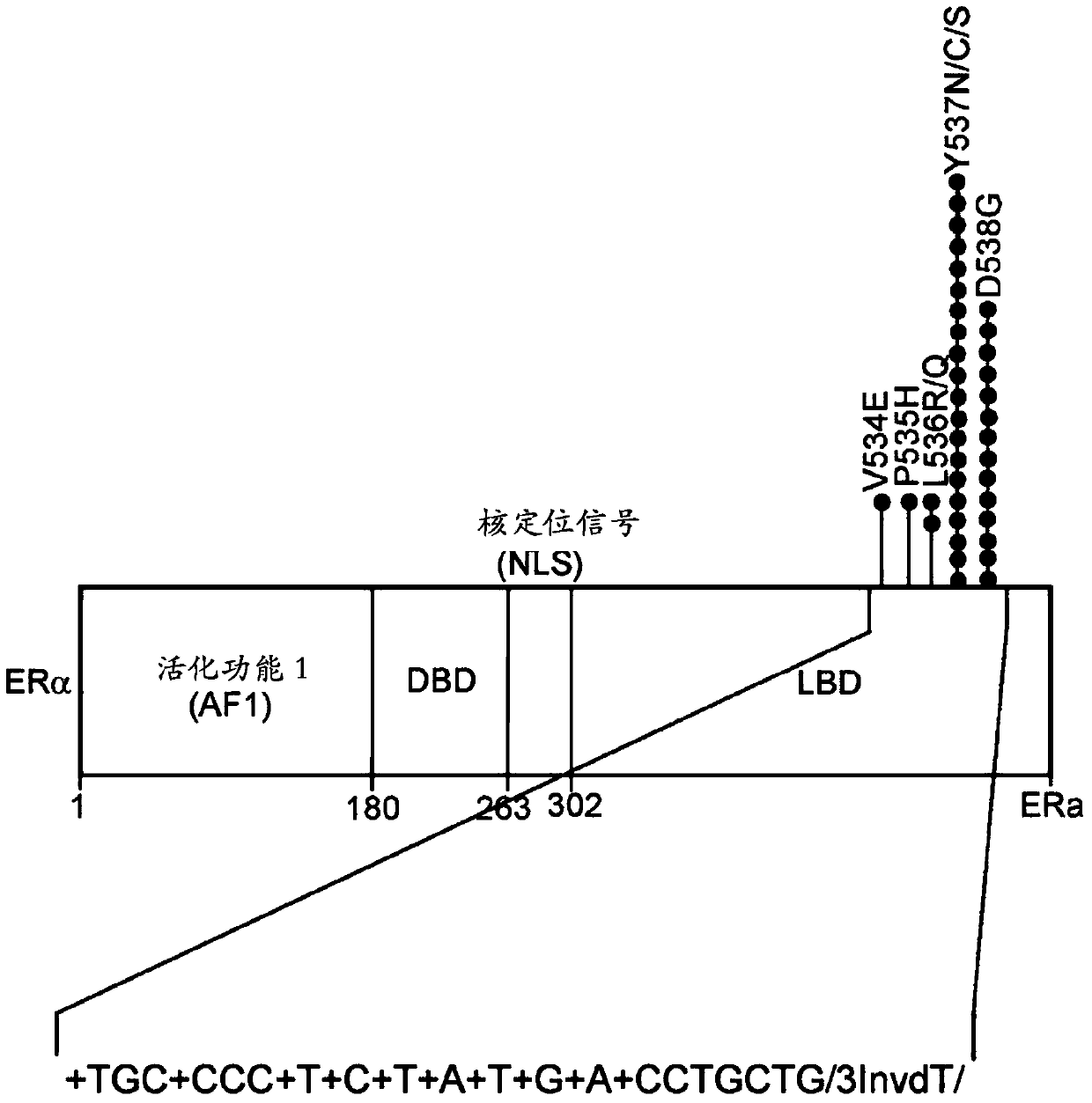
[0079] This example describes the development and exemplary implementation of the method described here as enrichment-sequencing, using locked nucleic acid clamps to recruit mutant-enriched set. A highly stringent, multiphase bioinformatics approach is then applied to ensure optimal specificity for mutation calling.
[0080] For the development of this technology, we first focused on estrogen receptor (ER)-positive breast cancers, in which recurrent mutations in the estrogen receptor alpha gene, ESR1, have recently been detected and appear to confer resistance to endocrine therapy (1-5). Identification of ESR1 mutations through noninvasive monitoring of women with metastatic breast cancer who have undergone endocrine therapy may allow early identification of treatment resistance, thereby allowing timely changes in therapy. Because mutations in ESR1 appear to cluster in the ligand-binding domai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com