Chiral alkane oxazinone compounds and their use as fungicides
A technology of butan and oxazinone, which is applied in the field of pesticides and can solve the problems of less research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
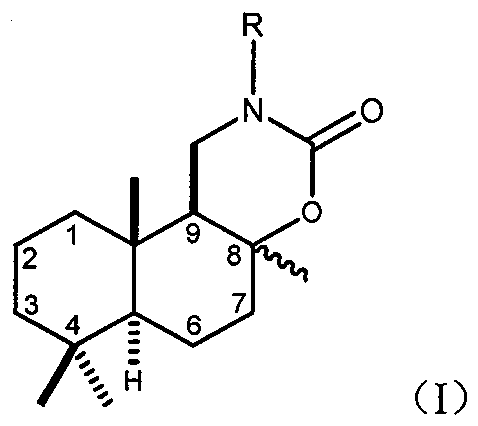
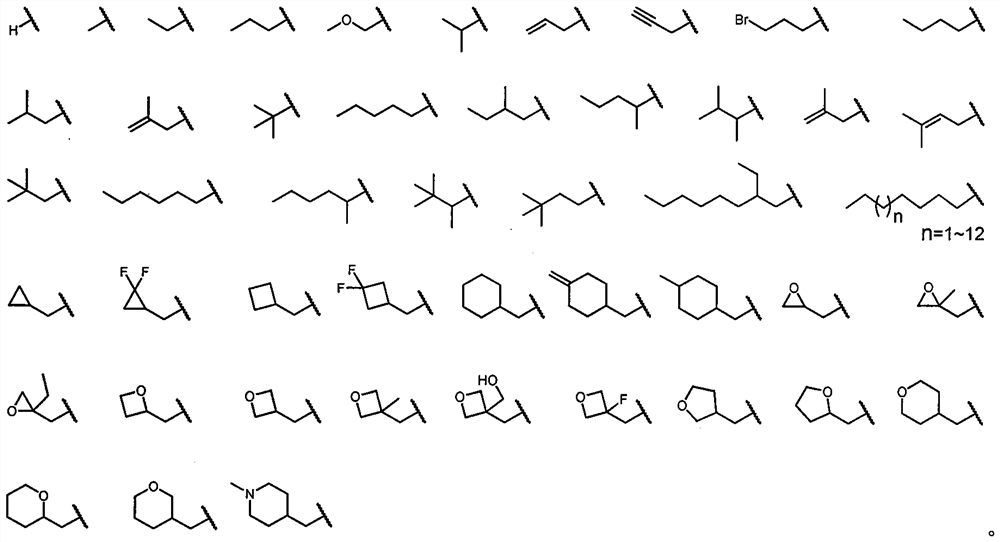
[0043] Example 1: (4aR, 6aS, 10aS, 10bS)-4a,7,7,10a-tetramethyldecalin-3H-naphtho[1,2-e][1,3]oxazine-3- Ketone synthesis
[0044]
[0045] The first step: the synthesis of ((+)-sclareolide) of sclareolide;
[0046] The natural product sclareol (-)-sclareol (10.0g, 32.4mmol, 1.0equiv) was dissolved in 200mL of anhydrous acetone, the system was placed in an ice bath, and 60mL of acetic anhydride was added thereto; Potassium manganate powder (30.7 g, 194.5 mol, 6 equiv). The system was gradually returned to room temperature, magnetically stirred, and thin layer chromatography (TLC) was used to track and detect the reaction process. After the raw material was consumed, an aqueous solution of sodium carbonate (20.0g / 150mL) was slowly added therein to quench the reaction, continued to stir for 0.5 hours, filtered, and the acetone was distilled off under reduced pressure to obtain the mixture of sclareolactone and licorane carboxylic acid. mixture.
[0047] 2N aqueous sodium h...
Embodiment 2
[0053] Example 2: (4aR, 6aS, 10aS, 10bS)-2-benzyl-4a,7,7,10a-tetramethyldecalin-3H-naphthyl[1,2-e][1,3] Synthesis of Oxazin-3-one
[0054]
[0055] Weigh the chiral alkoxazinone compound D (265 mg, 1.0 mmol, 1.0 equiv) and dissolve it in N, N-dimethylformamide solution (2 mL), add NaH (60% dispersion in mineral oil , 0.080g, 2.0mmol, 2.0equiv) and stirred the reaction at this temperature for 30 minutes, then added benzyl chloride (0.145mL, 1.2mmol, 1.2equiv), reacted under room temperature, thin layer chromatography (TLC) tracking detection reaction process. After the reaction was completed, the temperature was lowered to room temperature, and then water was added dropwise to quench the reaction. Add saturated ammonium chloride solution to neutralize to pH about 7, then extract with ethyl acetate 3 times (3x20mL), wash the organic phase with water in turn, wash with saturated sodium chloride, dry over anhydrous sodium sulfate, evaporate under reduced pressure Remove the ...
Embodiment 3
[0058]
[0059] Weigh chiral alkane oxazinone compound D (432mg, 1.6mmol, 1.0equiv) in a 50mL eggplant-shaped bottle, add dichloromethane solution (4mL), add methanol (250μL) in turn, trichloroisocyanuric acid (370mg, 1.6mmol, 1.0equiv), stirring reaction at room temperature for 4 hours (TLC monitors the reaction is complete), then flash silica gel column chromatography to obtain compound (4aR, 6aS, 10aS, 10bS)-2-chloro -4a,7,7,10a-Tetramethyldecalin-3H-naphthyl[1,2-e][1,3]oxazin-3-one, white solid, yield 96.4%, melting point 140.3- 140.7 (℃). 1 H NMR (400MHz, CDCl 3 ): δ3.71-3.63 (m, 1H, CH 2 N), 3.60 (dd, J=10.5, 6.1Hz, 1H, CH 2 N), 2.03(dt, J=12.9, 3.2Hz, 1H, CH-9), 1.97(dd, J=12.3, 6.1Hz, 1H), 1.77(d, J=14.2Hz, 1H), 1.63(ddd , J=25.6, 13.1, 7.5Hz, 3H), 1.48(dd, J=16.6, 6.1Hz, 3H), 1.43(s, 3H, CH 3 ), 1.36-1.24(m, 2H), 1.19(td, J=13.4, 3.7Hz, 1H), 1.09(dd, J=12.3, 3.5Hz, 1H), 1.01(dd, J=12.4, 1.9Hz, 1H), 0.91(s, 3H, CH 3 ), 0.90 (s, 3H, CH 3 ), 0.82 (s, 3H, CH 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com