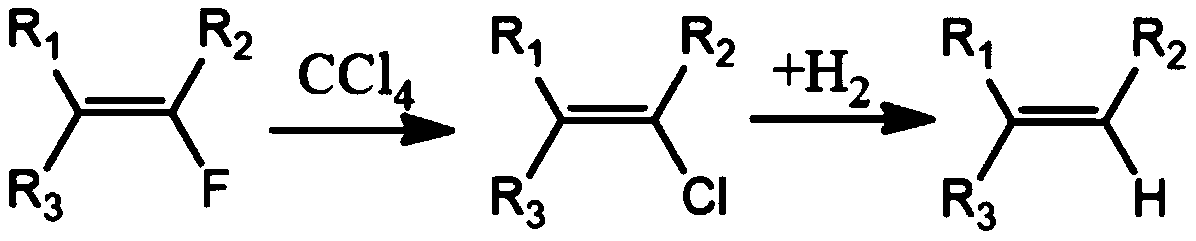
A kind of method for replacing fluorine on double bond of fluorine-containing olefin into hydrogen
A technology for fluorine-containing olefins and fluorine replacement, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve problems such as harsh reaction conditions, difficult preparation of raw materials, and restrictions on industrial production, and achieve cheap raw materials and three industrial wastes The effect of less and safe synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Preparation of the fluorine-to-chlorine catalyst used in the gas-phase fluorination reaction: the co-precipitation method is adopted, and the steps are as follows: CrCl 3 , Zn(NO 3 ) 2 , Mg(NO 3 ) 2 The solutions were mixed at a molar ratio of 70:20:10 to form a mixed solution, and then ammonia water accounting for 30% by weight of the above mixed solution was added dropwise to the above mixed solution, and the pH value of the mixed solution was adjusted to 9.0, after 48 hours of precipitation After aging, the precipitate was filtered and washed with deionized water, dried and pressed to form a Cr-Zn-Mg catalyst.
[0027] The drying process of the fluorine-to-chlorine catalyst: 20ml of Cr-Zn-Mg catalyst is loaded into the fixed-bed reactor, and then the fixed-bed reactor is heated by an open-type tube heating furnace. First, the Cr-Zn-Mg catalyst is raised to a temperature of 400°C at a rate of 10°C / min under the protection of 50ml / min of nitrogen and dried for ...
Embodiment 2
[0035] (1) Preparation of the fluorine-to-chlorine catalyst used in the gas-phase fluorination reaction: the co-precipitation method is adopted, and the steps are as follows: CrCl 3 , Mg(NO 3 ) 2 , Co(NO 3 ) 2 The solutions were mixed at a molar ratio of 70:20:10 to form a mixed solution, and then ammonia water accounting for 30% by weight of the above mixed solution was added dropwise to the above mixed solution, and the pH value of the mixed solution was adjusted to 9.0, after 48 hours of precipitation After aging, the precipitate was filtered and washed with deionized water, dried, and pressed to form a Cr-Mg-Co catalyst.
[0036] The drying process of the fluorine-to-chlorine catalyst: put 20ml of Cr-Mg-Co catalyst into the fixed-bed reactor, and then open the tube heating furnace to heat the fixed-bed reactor. First, the Cr-Cu-Zn catalyst is raised to a temperature of 400°C at a rate of 10°C / min under the protection of 50ml / min of nitrogen and dried for 10 hours, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com