Synthesis of phenylpyridazinone derivative and application thereof
A technology of pyridazine and phenyl, which is applied in the field of phenylpyridazine derivatives and their application in the treatment of mental and nervous diseases, and can solve the problems of QT gap prolongation, low ratio, EPS side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0100] The following examples are for the purpose of illustration only and are not intended to limit the invention.
[0101] A, the embodiment of synthetic aspect
Embodiment 1
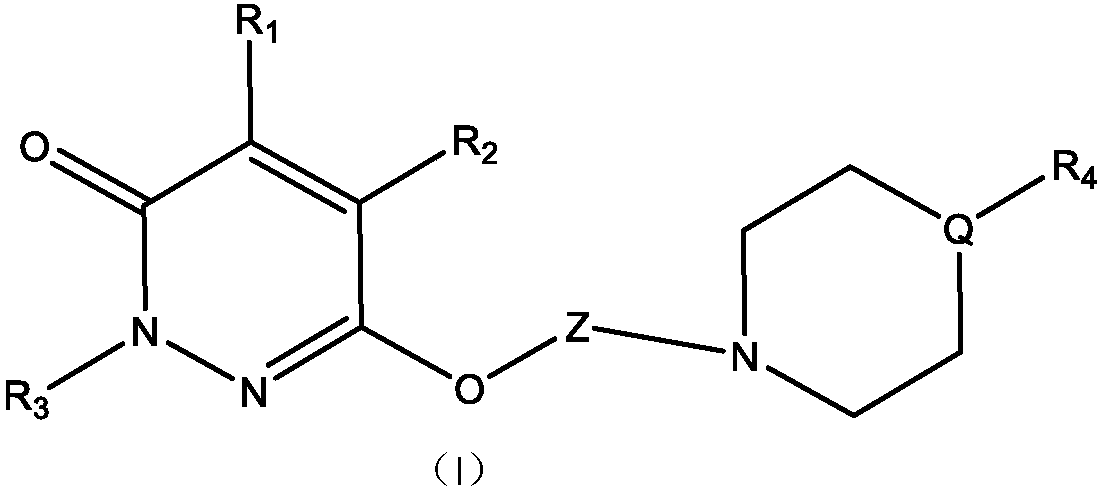
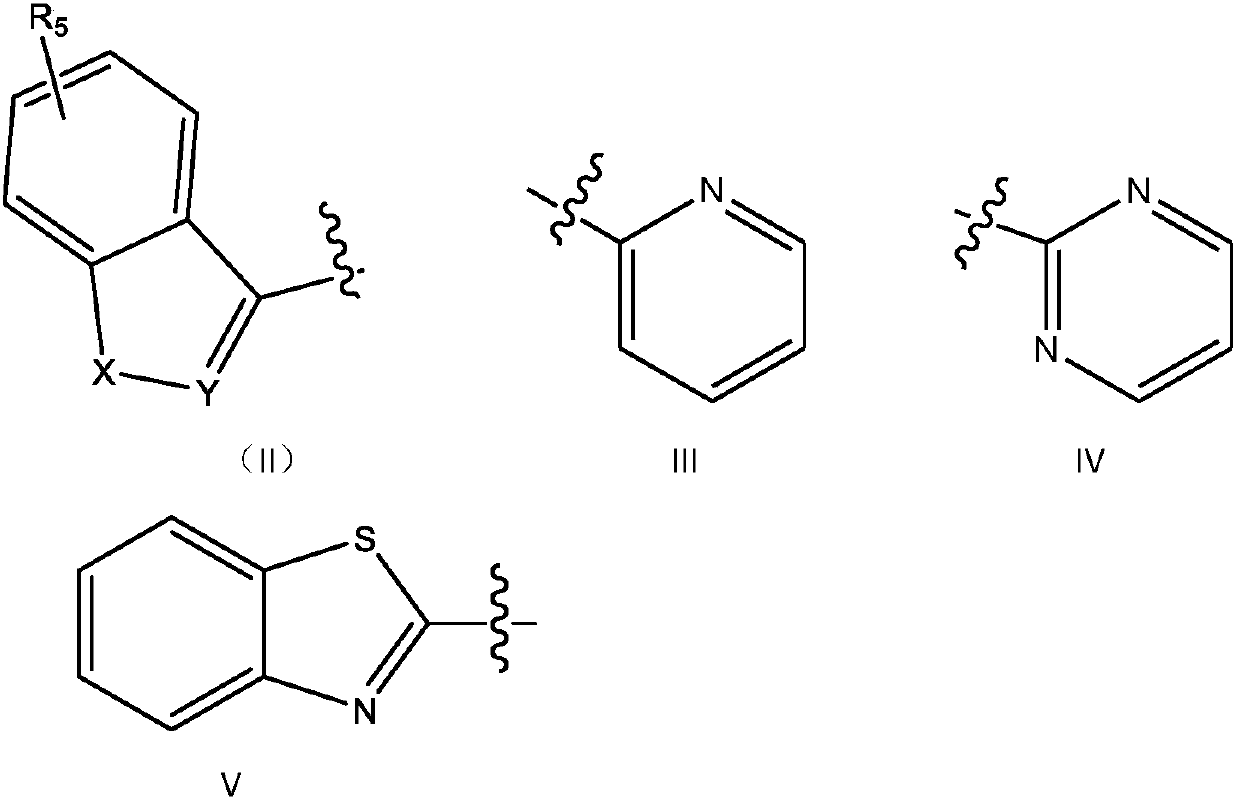
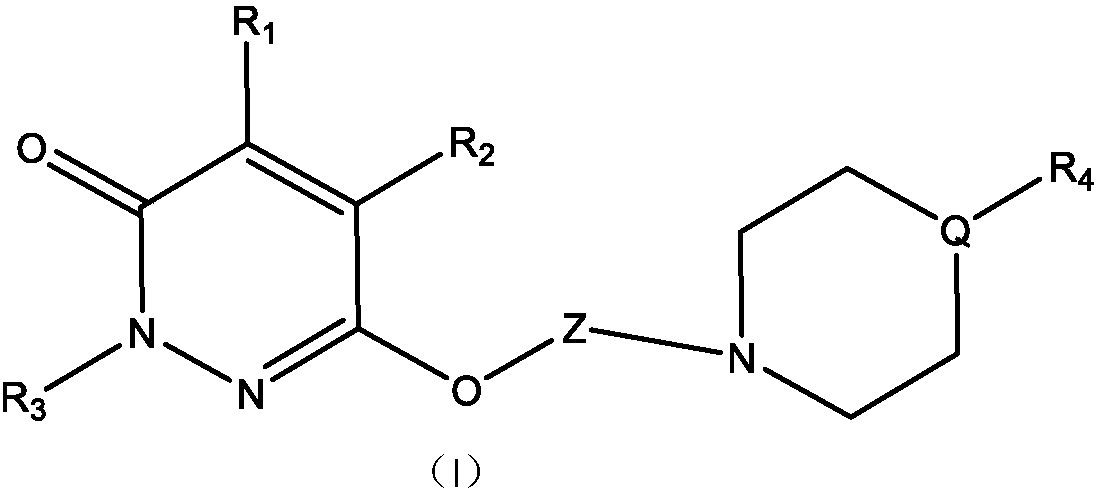
[0102] Example 1, 2-(3,4-dichlorophenyl)-6-(3-(4-(4-methoxyphenyl)piperazin-1-yl)propoxy)pyridazine-3( 2H)-one
[0103] Reaction 1
[0104]
[0105] 1) Dissolve 14.5 g of phenylhydrazine hydrochloride and 9.8 g of maleic anhydride in 200 ml of purified water. Under stirring, slowly add 40ml of concentrated hydrochloric acid, heat to reflux after the addition, and react for 6 hours. After the reaction was completed, the mixture was cooled in an ice-water bath, and a yellow solid was precipitated. Suction filtration, the filter cake was washed twice with water. Remove the filter cake with saturated NaHCO 3 After dissolving, the insoluble matter was filtered off, and the clear liquid was adjusted to a pH value between 2 and 3 with concentrated hydrochloric acid, and a white solid was precipitated, and 17.3 g was obtained after suction filtration and drying, with a yield of 92.0%.
[0106] 2) Take 9.4g of the first step product, 13.8g of anhydrous potassium carbonate, 18.8...
Embodiment 2
[0109] Example 2, 2-(3,4-dichlorophenyl)-6-(3-(4-(2-methoxyphenyl)piperazin-1-yl)propoxy)pyridazine-3( 2H)-one
[0110] Using 2-methoxyphenylpiperazine hydrochloride instead of 4-methoxyphenylpiperazine hydrochloride, the target compound was prepared according to the method of Example 1.
[0111] 1 H NMR (600MHz, CDCl 3 )δ7.89(d,J=2.5Hz,1H),7.65(dd,J=8.7,2.5Hz,1H),7.51(d,J=8.7Hz,1H),7.06–6.98(m,3H), 6.98–6.89(m,2H),6.87(dd,J=8.1,1.1Hz,1H),4.26(t,J=6.4Hz,2H),3.87(s,3H),3.12(s,4H),2.70 (s,4H),2.63–2.51(m,2H),2.09–1.94(m,2H).MS(ESI)m / z489.1([M+H] + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com