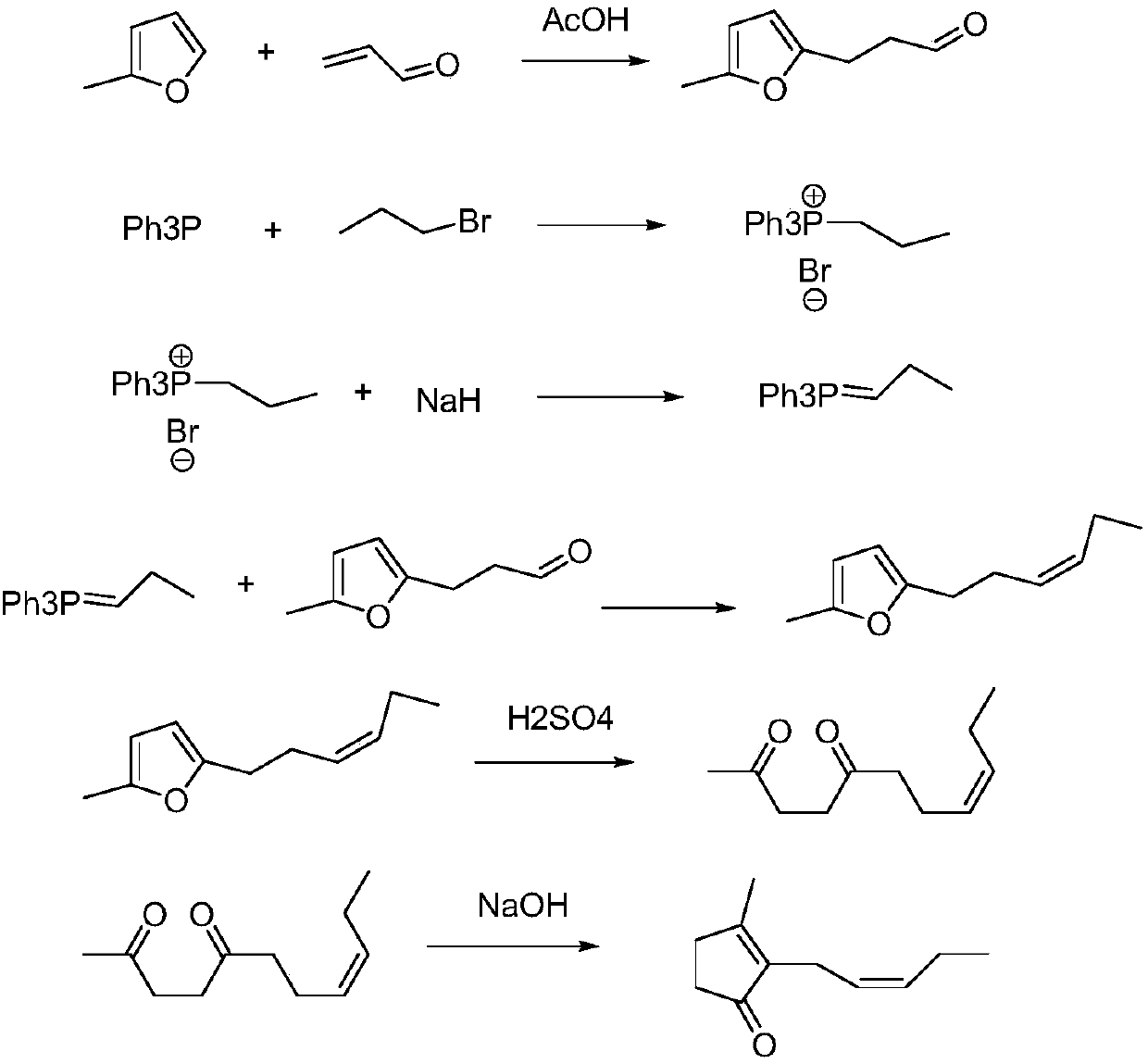
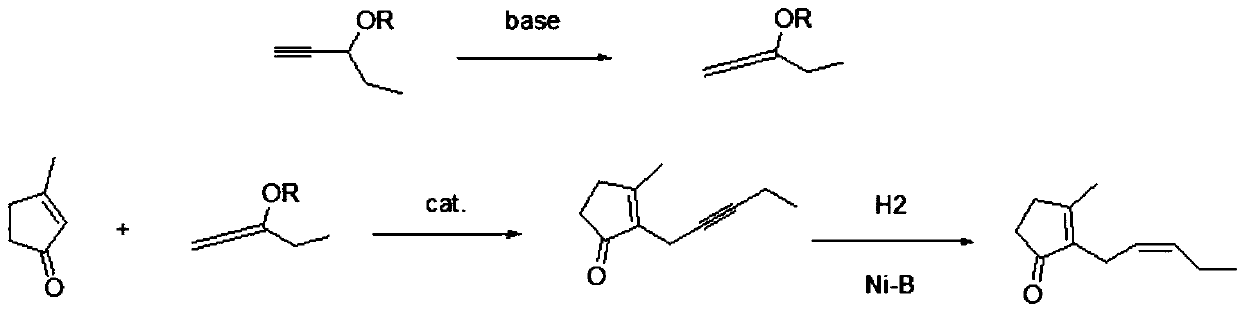
Preparation method of cis-jasmone
A technology of cis-jasmone and alkenone, which is applied in the field of preparation of cis-jasmone, can solve the problems of complex operation process, high preparation cost, and difficult process, and achieve simple preparation process, low process difficulty and low preparation. Favorable effects for mass production applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] see figure 2 , a preparation method of cis-jasmone, the specific preparation process is as follows:
[0027] (1) Preparation of 3-alkoxy-1,2-pentadiene (allene ether): the first prefabricated base and 3-alkoxy-1-pentyne are carried out according to the molar ratio of 0.001~0.1:1.0 Mix and carry out isomerization reaction at a temperature of 40° C. to 100° C. After 2 to 10 hours of reaction, 3-alkoxy-1,2-pentadiene is obtained.
[0028] Wherein, the first prefabricated base can be potassium tert-butoxide or potassium popentoxide, and 3-alkoxy-1,2-pentadiene can be 3-methoxy-1,2-pentadiene, 3- Ethoxy-1,2-pentadiene, 3-n-propoxy-1,2-pentadiene, 3-n-butoxy-1,2-pentadiene or 3-isobutoxy-1 , Any one of 2-pentadiene. It should also be pointed out here that, for the above-mentioned first preset temperature, the preferred range is 50° C. to 80° C.; for the above-mentioned first preset time, the preferred range is 4-8 hours.
[0029] (2) Preparation of 3-methyl-2-(2-pentynyl...
Embodiment 2
[0035] 1, Preparation of 3-methoxyl-1,2-pentadiene
[0036] In a 1000ml three-necked round-bottomed flask with a thermometer and a reflux condenser, add 687g of 3-methoxy-1-pentyne and 7.9g of potassium tert-butoxide, heat to 80°C, stir for 8 hours and then carry out normal pressure After distillation, 666 g of fractions at 110° C. to 112° C. were collected, corresponding to a yield of 97%.
[0037] 2. Preparation of 3-methyl-2-(2-pentynyl)-cyclopent-2-enone
[0038]In a 2000ml three-necked round bottom flask with a thermometer and a reflux condenser, add 192g of 3-methylcyclopent-2-enone, 255g of 3-methoxy-1,2-pentadiene, and 890g of tetrahydrofuran And 2.3g of 1,4-diazabicyclo[2.2.2]octane (DABCO), heated to reflux for 8h, cooled to room temperature, added 2.5g of benzoic acid, stirred at room temperature for 1h and then filtered, the filtrate first Recover tetrahydrofuran under normal pressure, then distill under reduced pressure, collect 107~110 ℃ / 1mmHg fraction, obtain ...
Embodiment 3
[0045] 1, Preparation of 3-methoxyl-1,2-pentadiene
[0046] In a 1000ml three-necked round bottom flask with a thermometer and a reflux condenser, add 687g of 3-methoxy-1-pentyne and 7.9g of potassium tert-butoxide, heat to 80°C, stir for 8 hours, and distill at atmospheric pressure , Collected 666g of fractions at 110-112°C, corresponding to a yield of 97%.
[0047] 2. Preparation of 3-methyl-2-(2-pentynyl)-cyclopent-2-enone
[0048] In a 2000ml three-neck round bottom flask with a thermometer and a reflux condenser, add 192g of 3-methylcyclopent-2-enone, 255g of 3-methoxy-1,2-pentadiene, and 890g of tetrahydrofuran And 4.0g of tri-n-butylphosphine, replace the air in the bottle with nitrogen three times, and heat to reflux for 8h. First recover tetrahydrofuran under normal pressure, then carry out vacuum distillation, collect 107~110 ℃ / 1mmHg fraction, obtain 282.3g of 3-methyl-2-(2-pentynyl)-cyclopent-2-enone, and collect The rate is 87%.
[0049] 3. Preparation of 3-met...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap