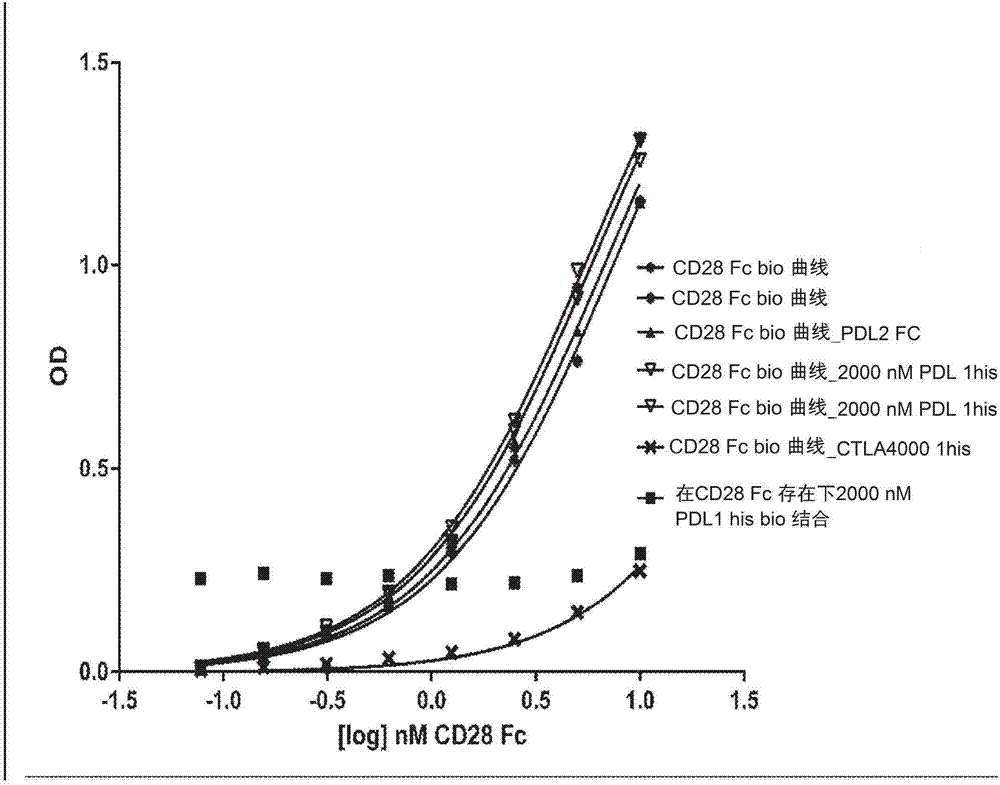
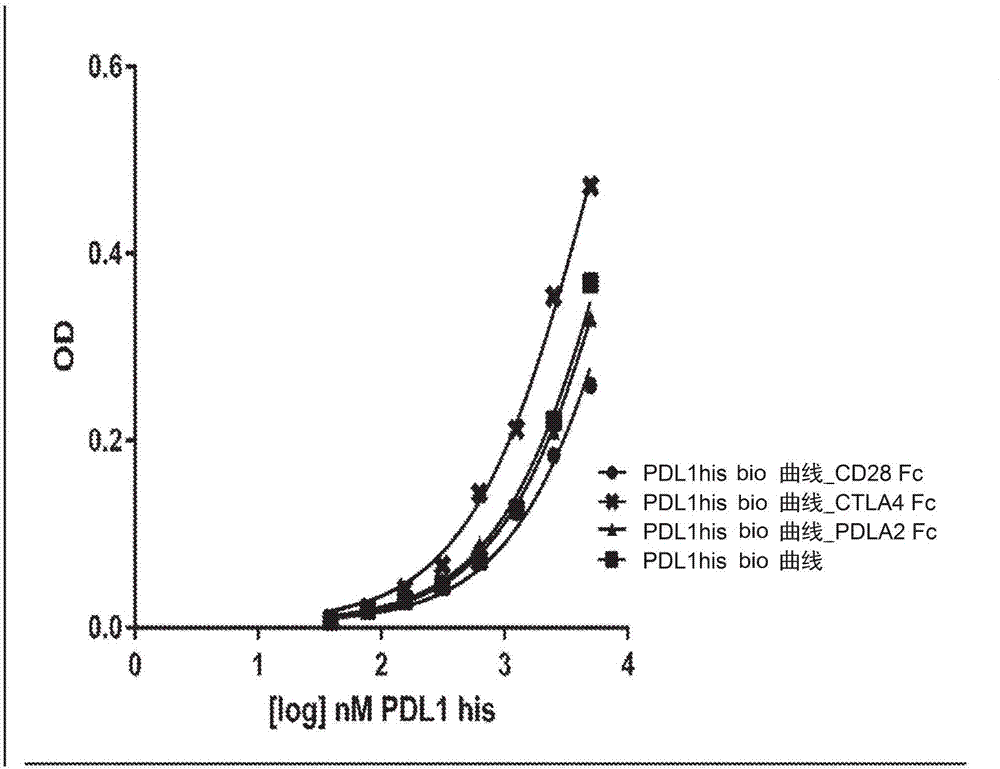
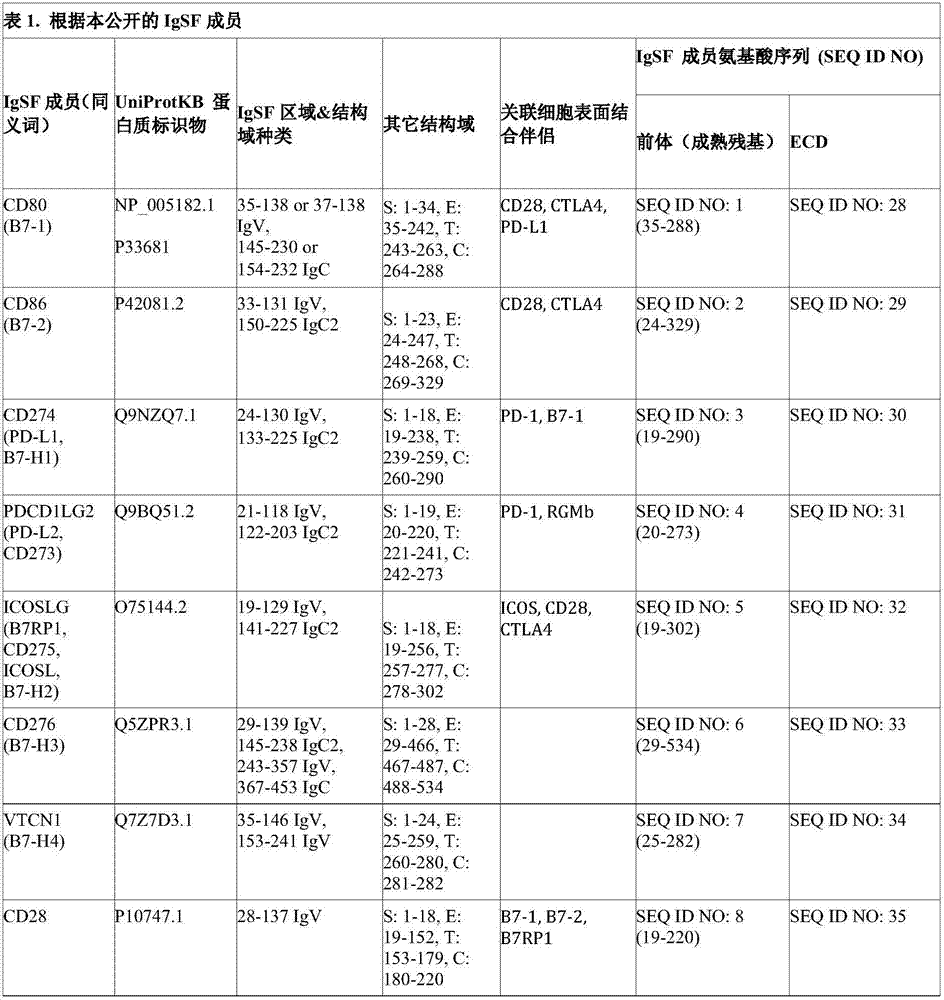
Immunomodulatory proteins with tunable affinities
A technology of immunomodulatory proteins and immunoglobulins, which can be used in peptide/protein components, animal/human proteins, medical preparations containing active ingredients, etc., and can solve problems such as lack of important attributes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0279] The implementation methods provided include:
[0280] Embodiment 1. In some embodiments, an immunomodulatory protein is provided, which contains at least one non-immunoglobulin affinity modified immunoglobulin superfamily (IgSF) domain, the immunoglobulin superfamily domain containing a wild-type IgSF domain One or more amino acid substitutions in wherein: at least one affinity-modified IgSF domain has enhanced binding to at least two associated binding partners compared to the wild-type IgSF domain; and at least one affinity-modified IgSF domain is specific To non-competitively bind at least two related binding partners.
[0281] Embodiment 2. In some other embodiments of embodiment 1, at least two cognate binding partners are cell surface molecules expressed on the surface of mammalian cells.
[0282] Embodiment 3. In some other embodiments of Embodiment 2, the cell surface molecule is expressed in a cis configuration or a trans configuration.
[0283] Embodiment 4. In s...
Embodiment 1
[0477] Generation of mutant DNA constructs of IgSF domain
[0478] Example 1 describes the generation of mutant DNA constructs of human CD80, CD86, ICOSL and NKp30 IgSF domains for translation and expression on the surface of yeast as a yeast display library.
[0479] A. Degenerate library
[0480] For libraries that are fully or partially randomized with degenerate codons for specific residues of the target protein, human CD80 (SEQ ID NO: 28), ICOSL (SEQ ID NO: 32) and NKp30 (SEQ ID NO: 54) cells A collection of overlapping oligonucleotides with a length of more than 80 base pairs (bp) from the coding DNA of the outer domain (ECD) was purchased from Integrated DNA Technologies (Clover, Iowa, USA). In order to generate a library of multiple variants of each ECD, the oligonucleotide contains the required degenerate codons at the required amino acid positions. Degenerate codons are generated using the algorithm located at URL: rosettadesign.med.unc.edu / SwiftLib / .
[0481] Generally, t...
Embodiment 2
[0488] DNA library introduced into yeast
[0489] Example 2 describes the introduction of CD80, CD86, ICOSL, and NKp30 DNA libraries into yeast.
[0490] In order to introduce degenerate and random library DNA into yeast, electroporation competent cells of yeast strain BJ5464 (ATCC.org; ATCC No. 208288) were prepared, and they were basically as described on Gene Pulser II (BioRad, USA). The electroporation is performed using the electroporation ready-to-use DNA from the above steps (Colby, DW, etc., 2004 Methods Enzymology 388, 348-358). The only exception is that the transformed cells were grown in non-inducible minimal selective SCD-Leu medium to accommodate the LEU2 selectable marker carried by the modified plasmid pBYDS03.
[0491] The library size was determined by plating a dilution of freshly recovered cells on SCD-Leu agar plates, and then inferring the library size from the number of individual colonies from the plating that produced at least 50 colonies per plate. The rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com