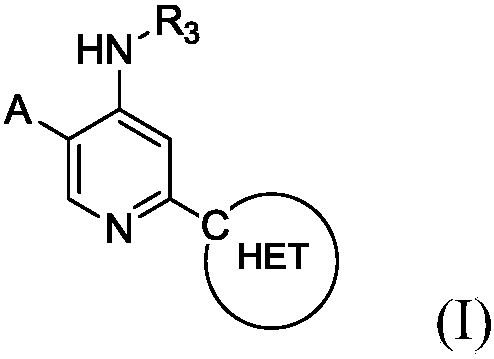
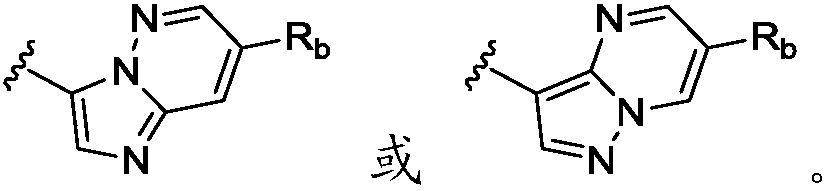
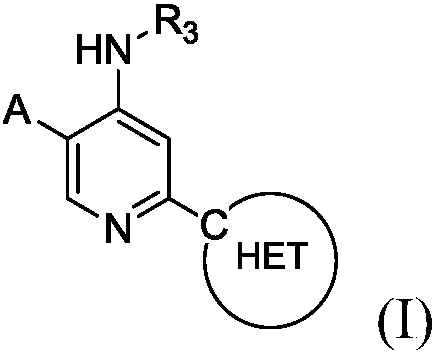
Heteroaryl substituted aminopyridine compounds
A compound, heteroaryl technology, applied in the field of aminopyridine compounds, can solve problems such as signal loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] 3-(5-(4-(2-hydroxyprop-2-yl)-1H-pyrazol-1-yl)-4-(isopropylamino)pyridin-2-yl)pyrazolo[1,5 -a]pyrimidine-6-carbonitrile
[0184]
[0185] Intermediate 1A: ethyl 1-(6-chloro-4-(isopropylamino)pyridin-3-yl)-1H-pyrazole-4-carboxylate
[0186]
[0187] To a stirred solution of 2-chloro-5-iodo-N-isopropylpyridin-4-amine (400 mg, 1.35 mmol) in 1,4-dioxane (10 mL) was added 1H-pyrazole-4-carboxy Ethyl acetate (189mg, 1.35mmol), CuI (51mg, 0.27mmol), K 2 CO 3 (373mg, 2.7mmol) and trans-N,N'-dimethylcyclohexyl-1,2-diamine (115mg, 0.81mmol). The mixture was sealed and heated at 110 °C for 14 hours. The solvent was removed and the mixture was partitioned between EtOAc and water. The organic layer was washed with Na 2 SO 4 Dry, filter, and concentrate. The product was purified by column chromatography (15% EtOAc / petroleum ether) to afford ethyl 1-(6-chloro-4-(isopropylamino)pyridin-3-yl)-1H-pyrazole-4-carboxylate ( 300 mg, 72% yield). LCMS 309.4 (M+H).
[0188] Inte...
Embodiment 2
[0200] 2-(7-Chlorimidazo[1,2-b]pyridazin-3-yl)-N-isopropyl-5-(4-(tetrahydro-2H-pyran-4-yl)-1H- Pyrazol-1-yl)pyridin-4-amine
[0201]
[0202] Intermediate 2A: 2-Chloro-5-iodopyridin-4-amine
[0203]
[0204] To a stirred solution of 2-chloropyridin-4-amine (5 g, 39 mmol) in DMF (50 mL) was added NIS (8.75 g, 39 mmol). The reaction mixture was then heated at 80°C for 3 hours. The mixture was cooled and DMF was removed in vacuo. The residue was partitioned between EtOAc and water and the layers were separated. The organic layer was washed with Na 2 SO 4 Dry, filter, and concentrate. The product was purified via column chromatography (10% EtOAc / petroleum ether) to afford 2-chloro-5-iodopyridin-4-amine (4 g, 39% yield). 1 H NMR (400MHz, DMSO-d 6 )δ8.20(s,1H),6.64(s,1H),6.50(br s,2H); LC / MS: 254.8(M + ). Further elution with 12% EtOAc / petroleum ether afforded 2-chloro-3-iodopyridin-4-amine (4 g, 39% yield).
[0205] Intermediate 2B: 2-Chloro-5-iodo-N-isopropylpyrid...
Embodiment 11
[0230] 2-(6-Chloropyrazolo[1,5-a]pyrimidin-3-yl)-N-isopropyl-5-(4-propyl-1H-1,2,3-triazole-1- base) pyridin-4-amine
[0231]
[0232] Intermediate 11A: 5-Bromo-2-chloro-N-isopropylpyridin-4-amine
[0233]
[0234] 5-Bromo-2,4-dichloropyridine (3.0 g, 13.22 mmol), isopropylamine (1.7 mL, 19.83 mmol) and Huenig base (11.6 mL, 66.1 mmol) in DMF (5 mL) were stirred at room temperature The solution in was then heated behind a hood at 120° C. for 4 hours, at which point it was judged complete by LCMS. The reaction mixture was diluted with ethyl acetate and washed with 10% LiCl (3 times). The organic layer was washed with Na 2 SO 4 Dry, filter and concentrate to give crude material. The product was purified by column chromatography (hexane / EtOAc) to give 5-bromo-2-chloro-N-isopropylpyridin-4-amine (1.29 g, 37% yield) as a colorless oil. LCMS m / z 249.0 (M+H).
[0235] Intermediate 11B: 2-Chloro-N-isopropyl-5-(4-propyl-1H-1,2,3-triazol-1-yl)pyridin-4-amine
[0236]
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com