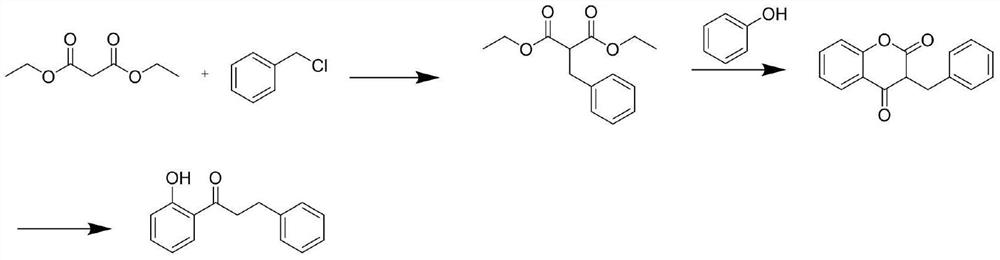
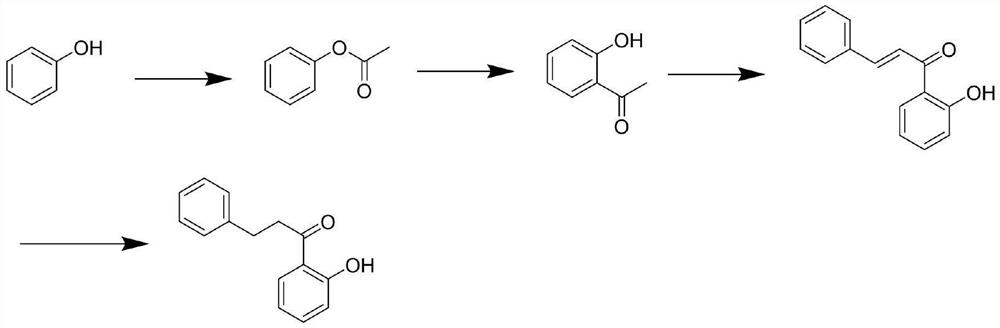
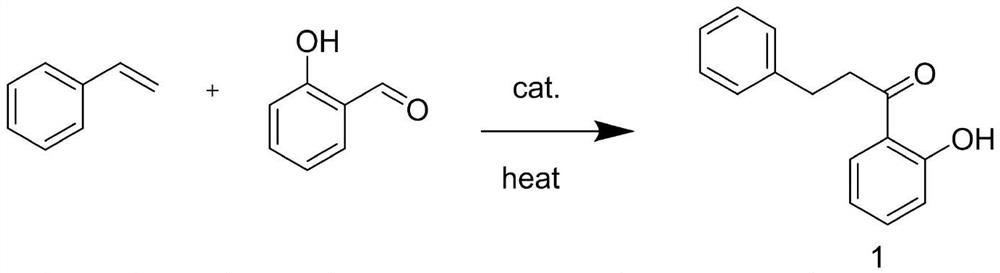
A kind of preparation method of 2-hydroxyphenylpropiophenone
A technology of hydroxyphenylbenzene and hydroxybenzaldehyde, applied in the field of medical research, can solve problems such as difficult separation, difficult operation, and low yield, and achieve the effects of simple treatment, shortened reaction time, and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] in N 2 Under protection, in a 3000ml four-neck flask, add 1500ml dichloroethane, 124g styrene and 122g salicylaldehyde, then add 5mol% PdCl 2 And 5mol% potassium phosphate, heated to 75 ° C for 6.5 hours. Cool down to room temperature, filter with suction, and wash the catalyst with a small amount of dichloroethane. The filtrate was evaporated to dryness to obtain a light yellow viscous liquid. Distilled under reduced pressure to obtain 194 g of a colorless transparent liquid with a yield of 85.8%.
Embodiment 2
[0031] in N 2 Under protection, in a 3000ml four-neck flask, add 1500ml dichloroethane, 124g styrene and 122g salicylaldehyde, then add 2.5mol% PdCl 2 And 2.5mol% potassium phosphate, heated to 75 ° C for 6.5 hours. Cool down to room temperature, filter with suction, and wash the catalyst with a small amount of dichloroethane. The filtrate was evaporated to dryness to obtain a light yellow viscous liquid. Distilled under reduced pressure to obtain 186 g of a colorless transparent liquid with a yield of 82.3%.
Embodiment 3
[0033] in N 2 Under protection, in a 3000ml four-neck flask, add 1500ml dichloroethane, 124g styrene and 122g salicylaldehyde, then add 5mol% PdCl 2 (the seventh recovery) and 5mol% potassium phosphate, the temperature was raised to 75° C. for 6.5 hours. Cool down to room temperature, filter with suction, and wash the catalyst with a small amount of dichloroethane. The filtrate was evaporated to dryness to obtain a light yellow viscous liquid. Distilled under reduced pressure to obtain 189 g of a colorless transparent liquid with a yield of 83.6%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap