Use of chlorite to treat red blood cell diseases and indications mediated thereby
A chlorite, diabetes technology, applied in extracellular fluid diseases, metabolic diseases, blood diseases, etc., can solve problems such as no evidence of inhibition of AGE end-organ damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0080] A preferred composition is WF10, eg, disclosed in US Patent No. 8,252,343 as an agent for the treatment of allergies, asthma and dermatitis.
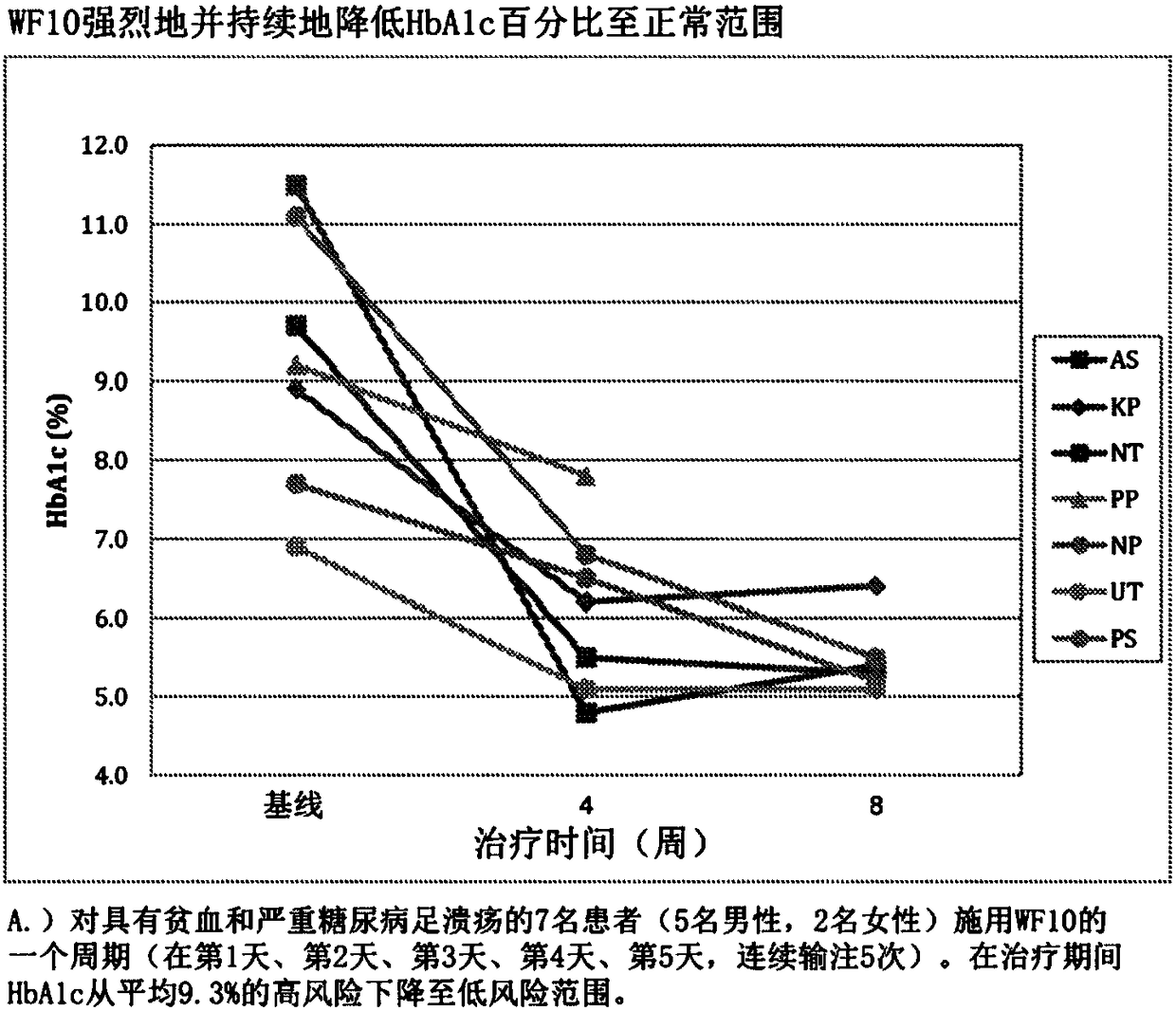
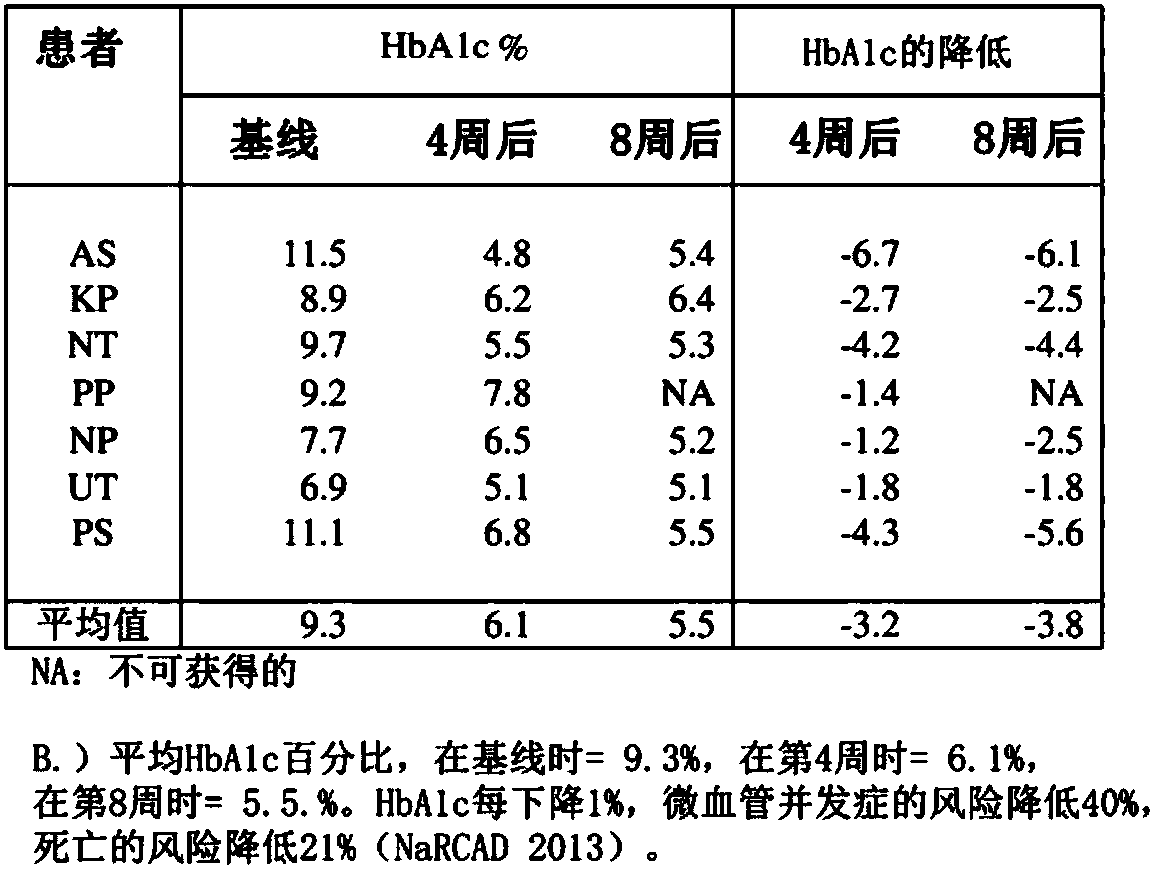
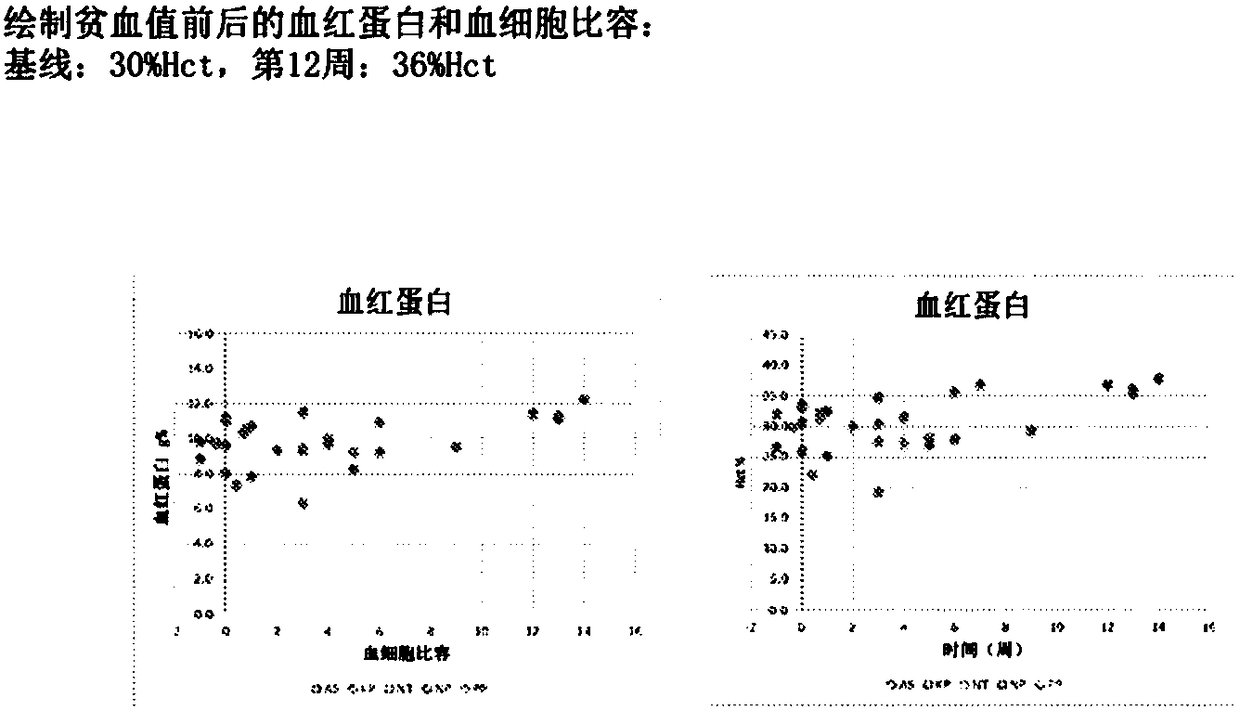
[0081] WF10 is a sterile, pyrogen-free, 10% (w / v) dilute aqueous solution of the drug OXO-K993, which is analytically characterized as containing ionic chlorite (4.25%), chloride (2.0%), chlorate (1.5%), sulfate (0.7%) and sodium (4.0%) solution. Human clinical research has generated a large amount of safety evidence. When WF10 is infused at a daily dose of 0.5mL / kg for 5 consecutive days, followed by a 16-day drug-free interval, it constitutes a "cycle". In both regimens, patients received 6 cycles of treatment, and in another trial, patients received 4 cycles followed by maintenance every 6 weeks for up to 128 weeks. In any event, WF10 showed an excellent safety profile, with no steroid-like side effects, no immunosuppression, no antihistamine-like side effects, and no cardiovascular side effects.
[0082] WF10 has been shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com