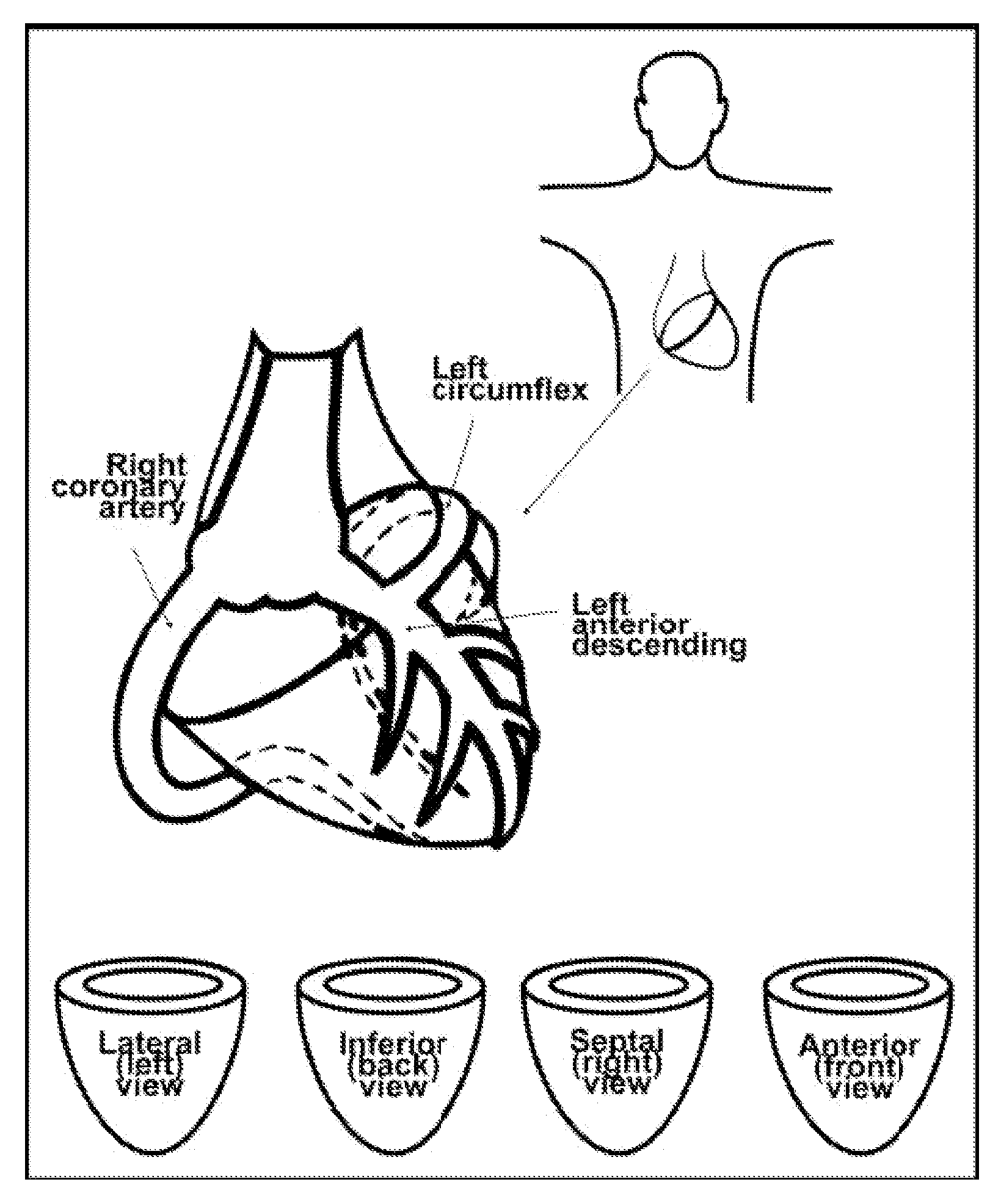
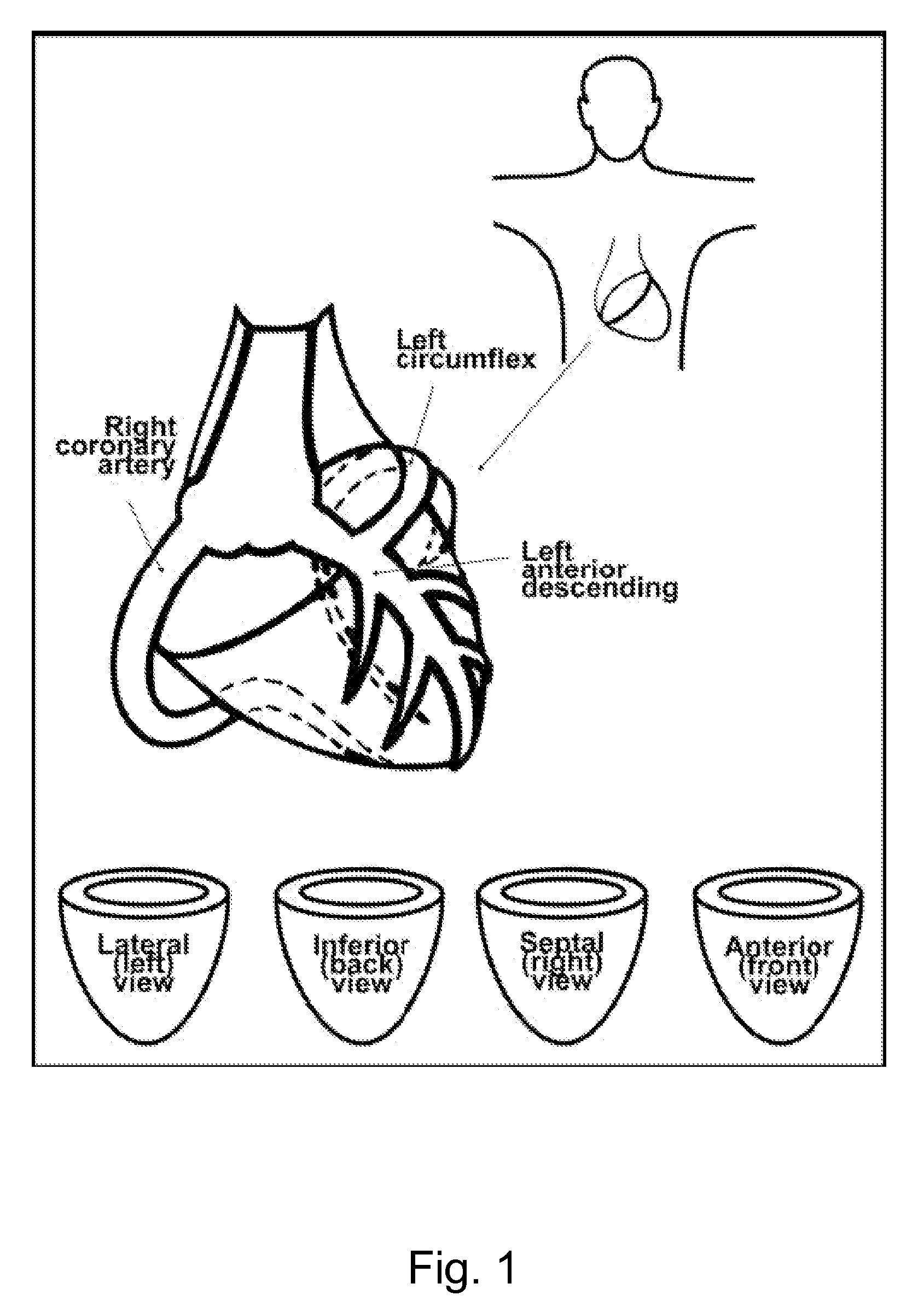
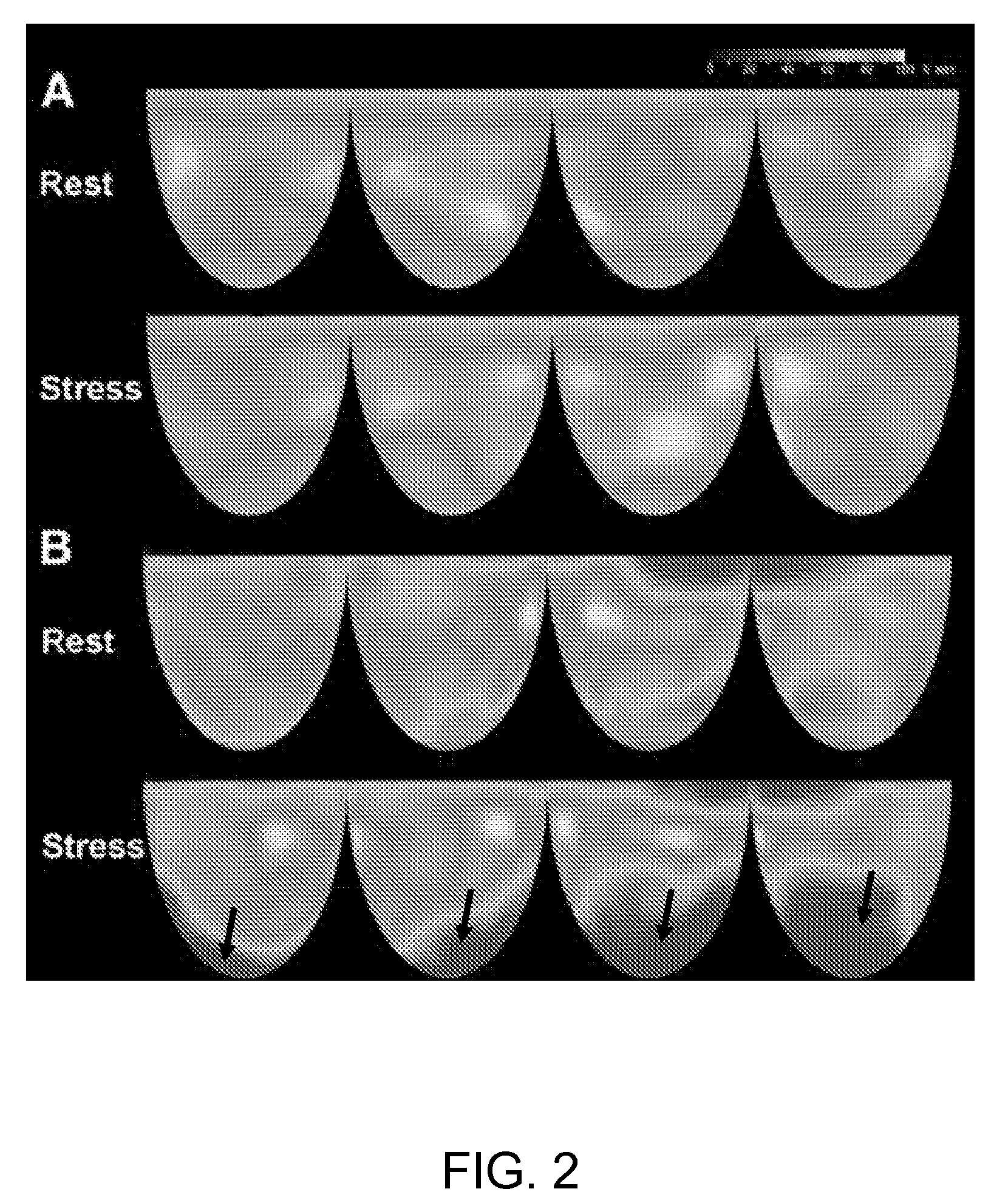
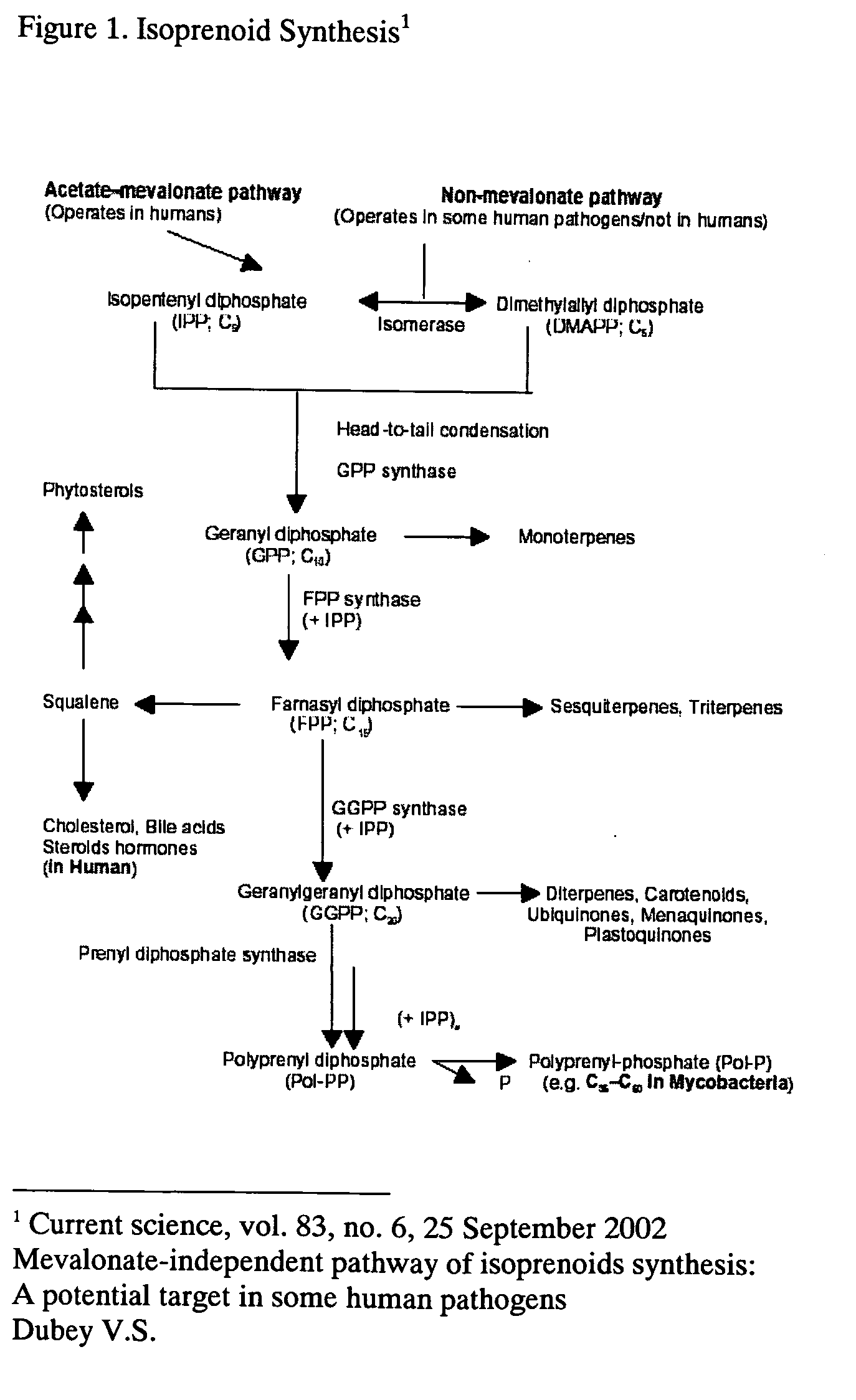
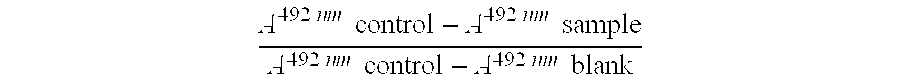Patents
Literature
86 results about "Coronary vascular disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Coronary artery disease — also called coronary vascular, arteriosclerotic and ischemic heart disease — remains the leading cause of death in the United States. The disease is caused by arteriosclerosis or "hardening of the arteries," which interferes with the normal flow of blood to the heart.
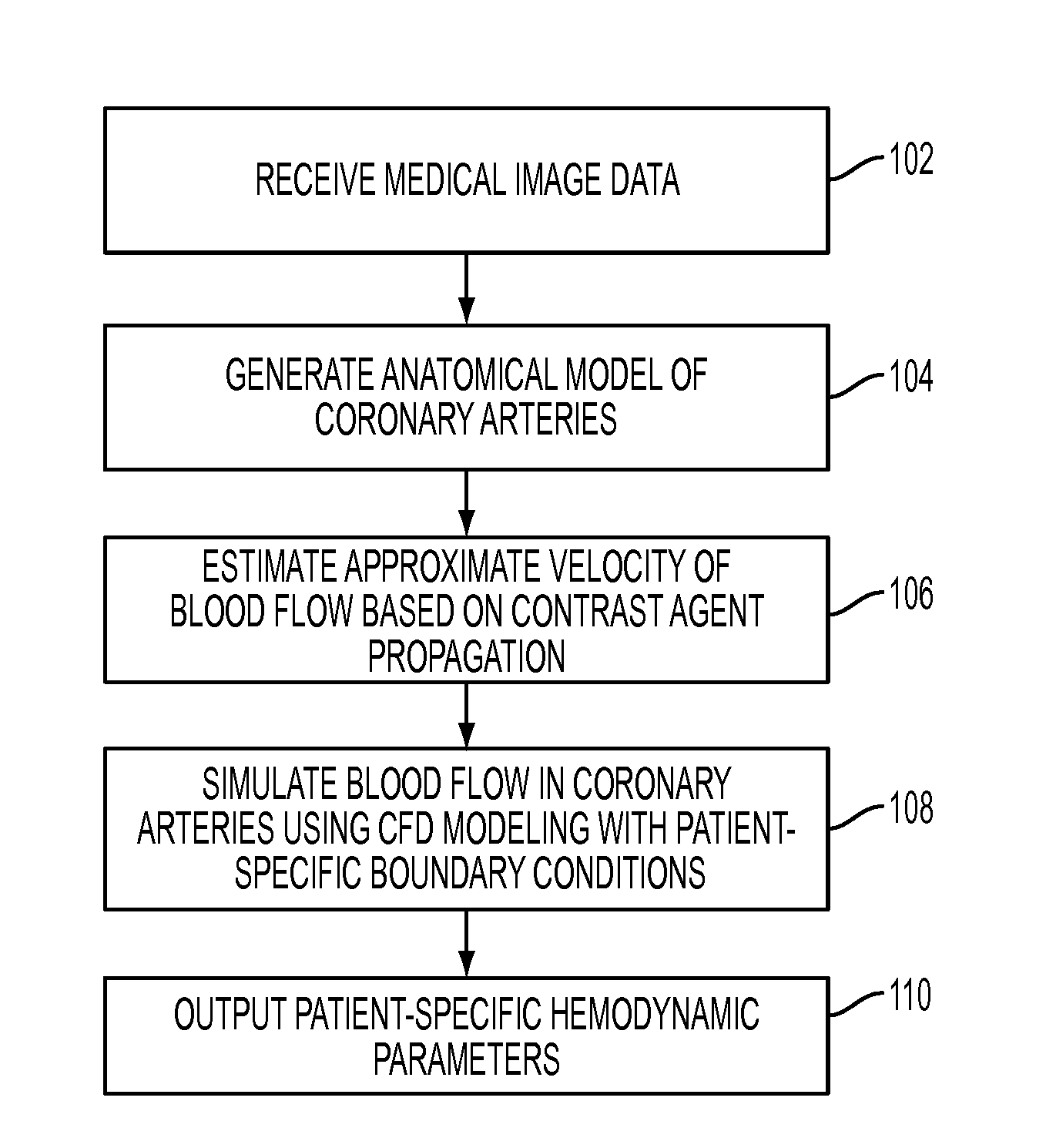
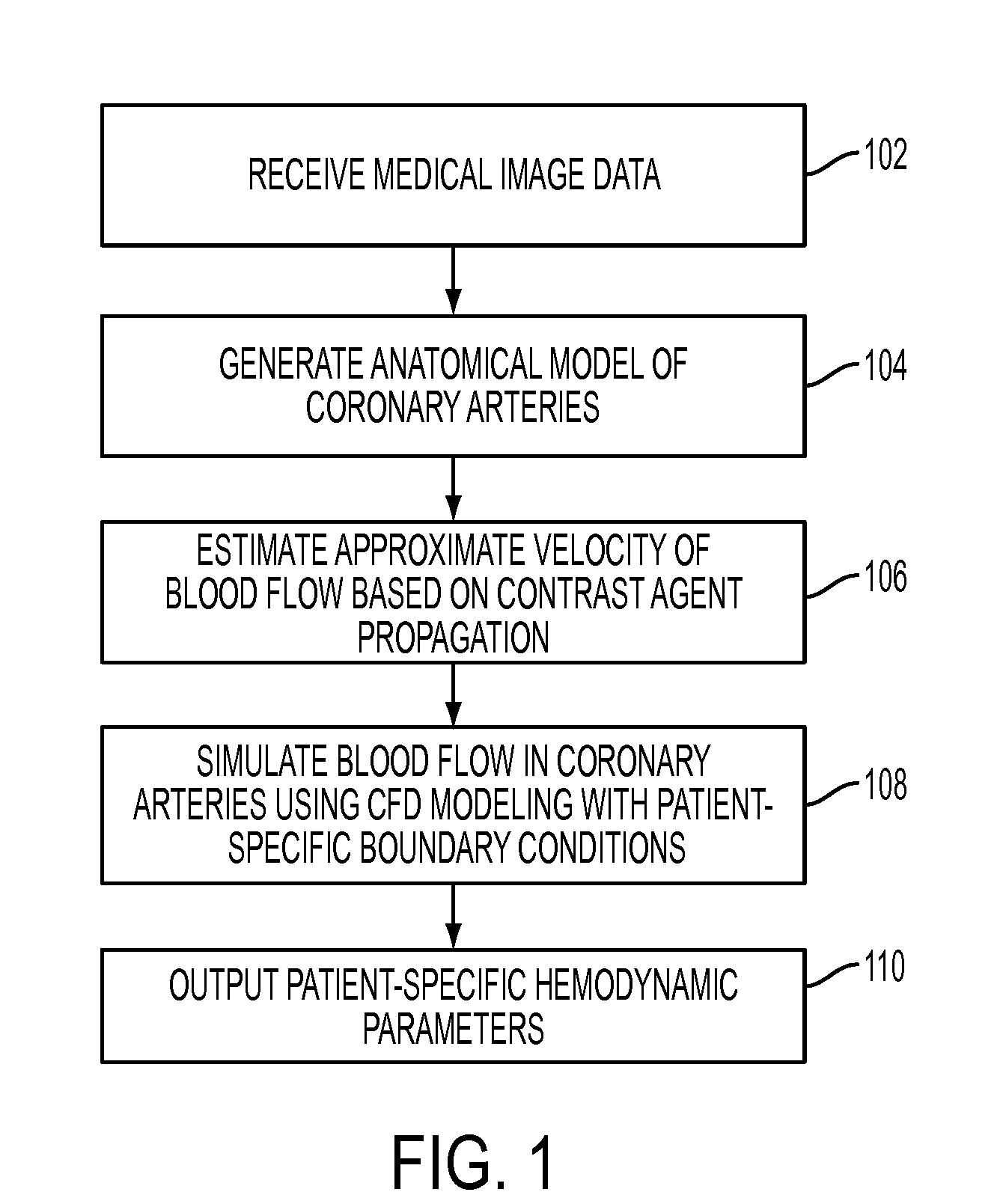
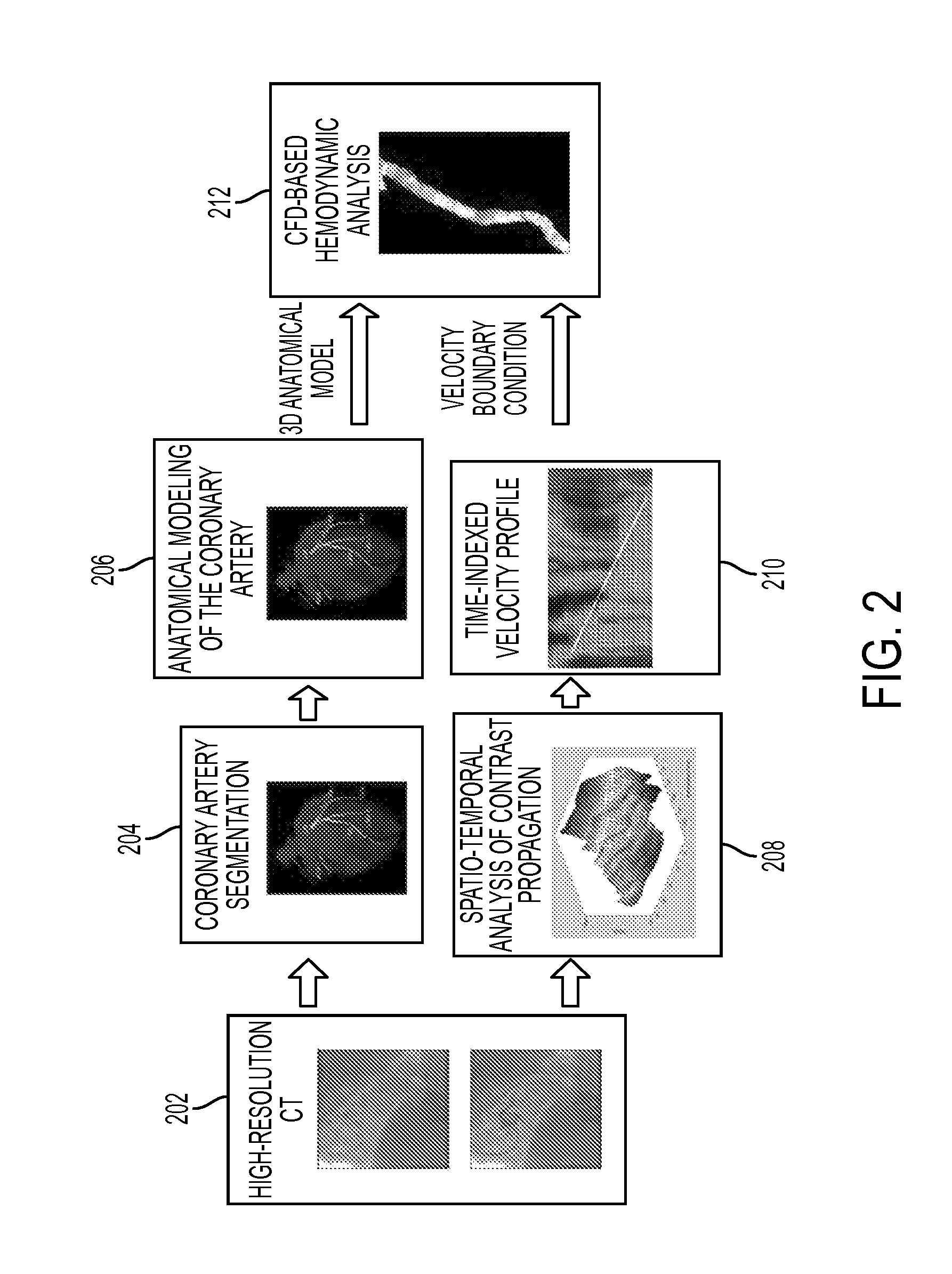
Method and System for Non-Invasive Assessment of Coronary Artery Disease
A method and system for non-invasive patient-specific assessment of coronary artery disease is disclosed. An anatomical model of a coronary artery is generated from medical image data. A velocity of blood in the coronary artery is estimated based on a spatio-temporal representation of contrast agent propagation in the medical image data. Blood flow is simulated in the anatomical model of the coronary artery using a computational fluid dynamics (CFD) simulation using the estimated velocity of the blood in the coronary artery as a boundary condition.
Owner:SIEMENS HEALTHCARE GMBH
Catheter devices and methods for their use in the treatment of calcified vascular occlusions
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Method of prevention and treatment of aging and age-related disorders including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimer's disease and cancer
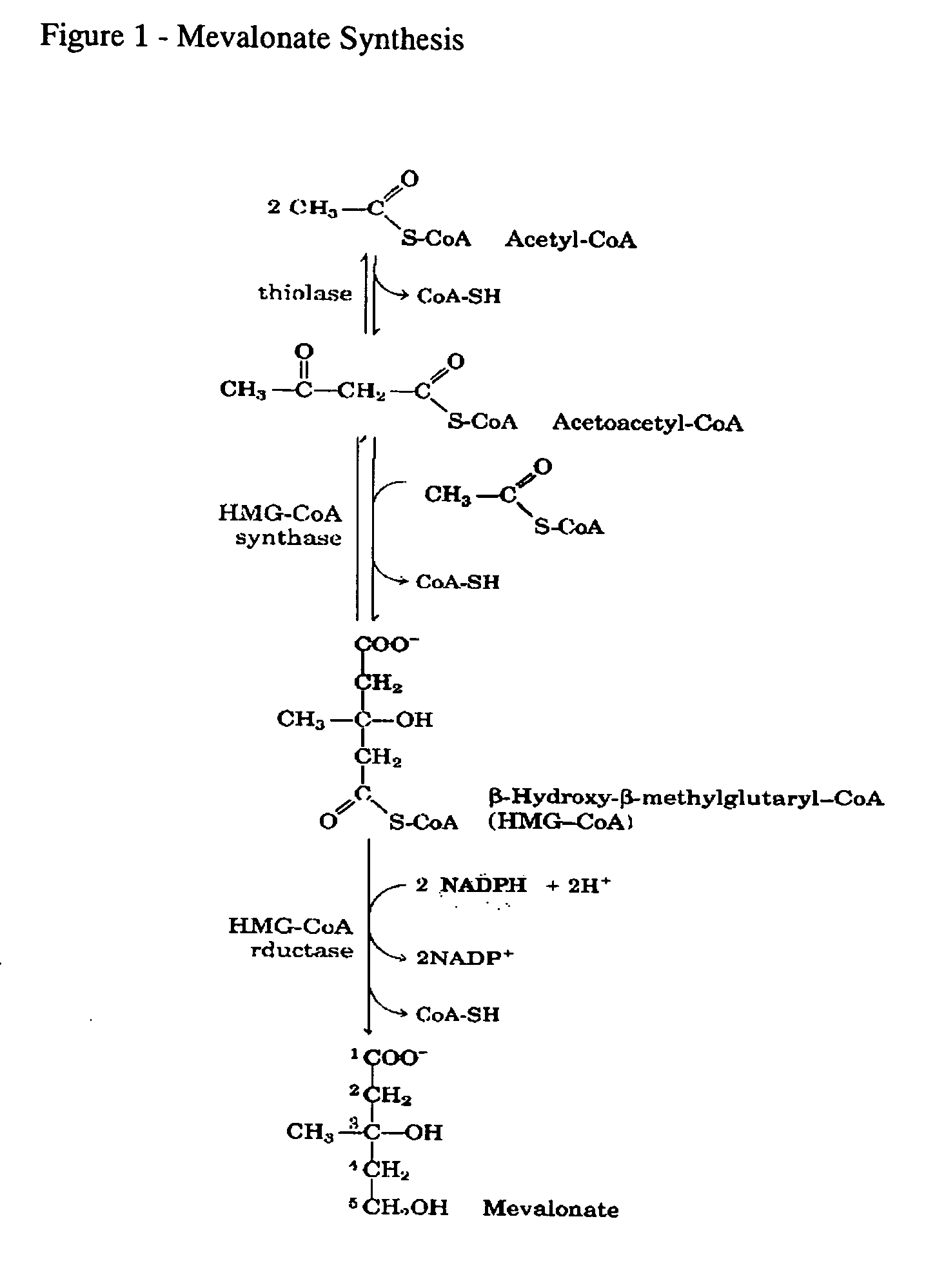
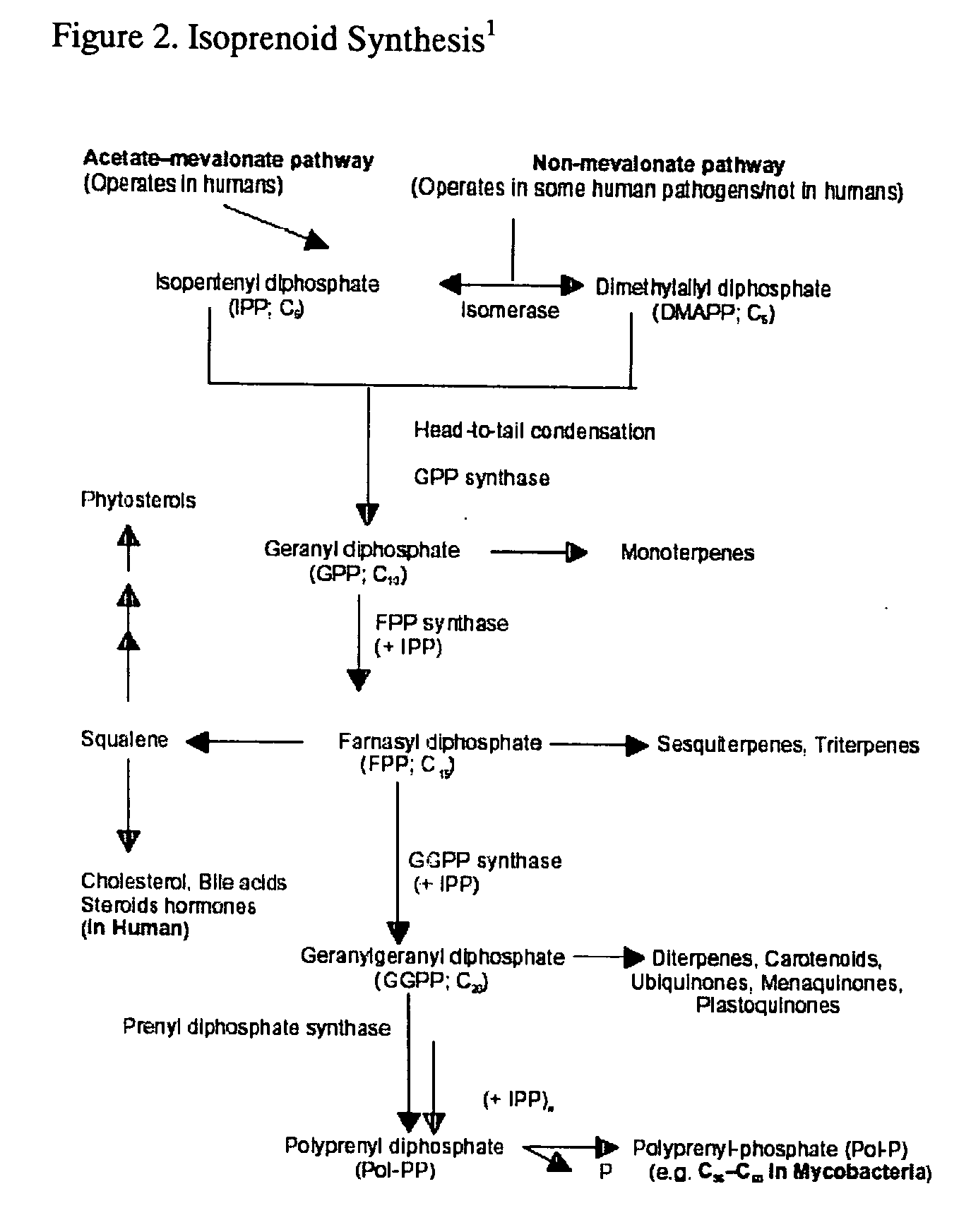
This invention relates to a method for prevention and treatment of aging and age-related disorders including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, type 2 diabetes, dementia and some forms of arthritis and cancer in a subject comprising administering to said subject, separately, sequentially or simultaneously a therapeutically effective dosage of each component or combination of statins, bisphosphonates, cholesterol lowering agents or techniques, interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof, administered separately, in sequence or simultaneously. Inhibition of the signal transduction pathway for Interleukin 6 mediated inflammation is key to the prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, aging and age-related disorders including osteoporosis, type 2 diabetes, dementia and some forms of arthritis and tumors. Inhibition of Interleukin 6 mediated inflammation may be achieved indirectly through regulation of endogenous cholesterol synthesis and isoprenoid depletion or by direct inhibition of the signal transduction pathway utilizing interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism, isoprenoid depletion and / or inhibition of the signal transduction pathway.
Owner:OMOIGUI OSEMWOTA SOTA
Method for detecting coronary endothelial dysfunction and early atherosclerosis
InactiveUS20080058642A1Reduce incidenceReduce riskTomographyAngiographyTherapeutic treatmentCoronary Artery Disease Risk
A method of detecting endothelin receptor A mediated coronary microvascular endothelial dysfunction in an asymptomatic subject is disclosed. The method comprises obtaining sets of noninvasive cardiac PET perfusion images of the subject before and after administration of selective endothelin receptor A (ETA receptor) antagonist. The images are analyzed, including application of applying Markovian homogeneity analysis, and the results are compared to detect improvement, or lack of improvement, of myocardial perfusion homogeneity in the subject. A result of improved myocardial perfusion homogeneity after administration of the antagonist indicates the presence of ETA receptor-mediated microvascular endothelial dysfunction in the subject and indicates therapeutic treatment to improve endothelial function and / or to reduce coronary artery disease risk factors.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method of prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, and age-related disorders including osteoporosis, arthritis, type 2 diabetes, dementia and Alzheimer's disease
InactiveUS20060078531A1Avoid problemsBiocidePhosphorous compound active ingredientsInterleukin 6Age related disease
This invention relates to a method for prevention and treatment of Atherosclerosis, Peripheral Vascular Disease, Coronary Artery Disease, and age-related disorders including Osteoporosis, Arthritis, Type II Diabetes, Dementia and Alzheimer's disease in a subject comprising administering to said subject a therapeutically effective dosage of each component or combination of statins, bisphosphonates and / or cholesterol lowering agents or techniques, administered separately, in sequence or simultaneously. Cholesterol Metabolites (isoprenoids) are an integral component of the signaling pathway for Interleukin 6 mediated inflammation. Interleukin 6 inflammation is the common causative origin for Atherosclerosis, Peripheral Vascular Disease, Coronary Artery Disease, and age-related disorders including Osteoporosis, Arthritis, Type II Diabetes, Dementia and Alzheimer's disease. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism and isoprenoid depletion.
Owner:SOTA OSEMWOTA
Detection of asymptomatic coronary artery disease using atherogenic proteins and acute phase reactants
InactiveUS20050181451A1Easy to detectDelay disease progressionDisease diagnosisBiological testingCoronary arteriesCoronary Artery Disease Symptoms
A method of assessing the likelihood that a person who is asymptomatic for coronary artery disease does in fact have the disease is disclosed. The levels of an atherogenic protein and acute phase reactant and optionally of anti-atherogenic protein for an individual are obtained and compared to one or more cut-points related to those substances and, based on the comparison(s), an assessment is made of the likelihood that the individual has coronary artery disease. The atherogenic protein may be OxLDL, the acute phase reactant may be C-reactive protein or fibrinogen, and the anti-atherogenic protein may be HDL.
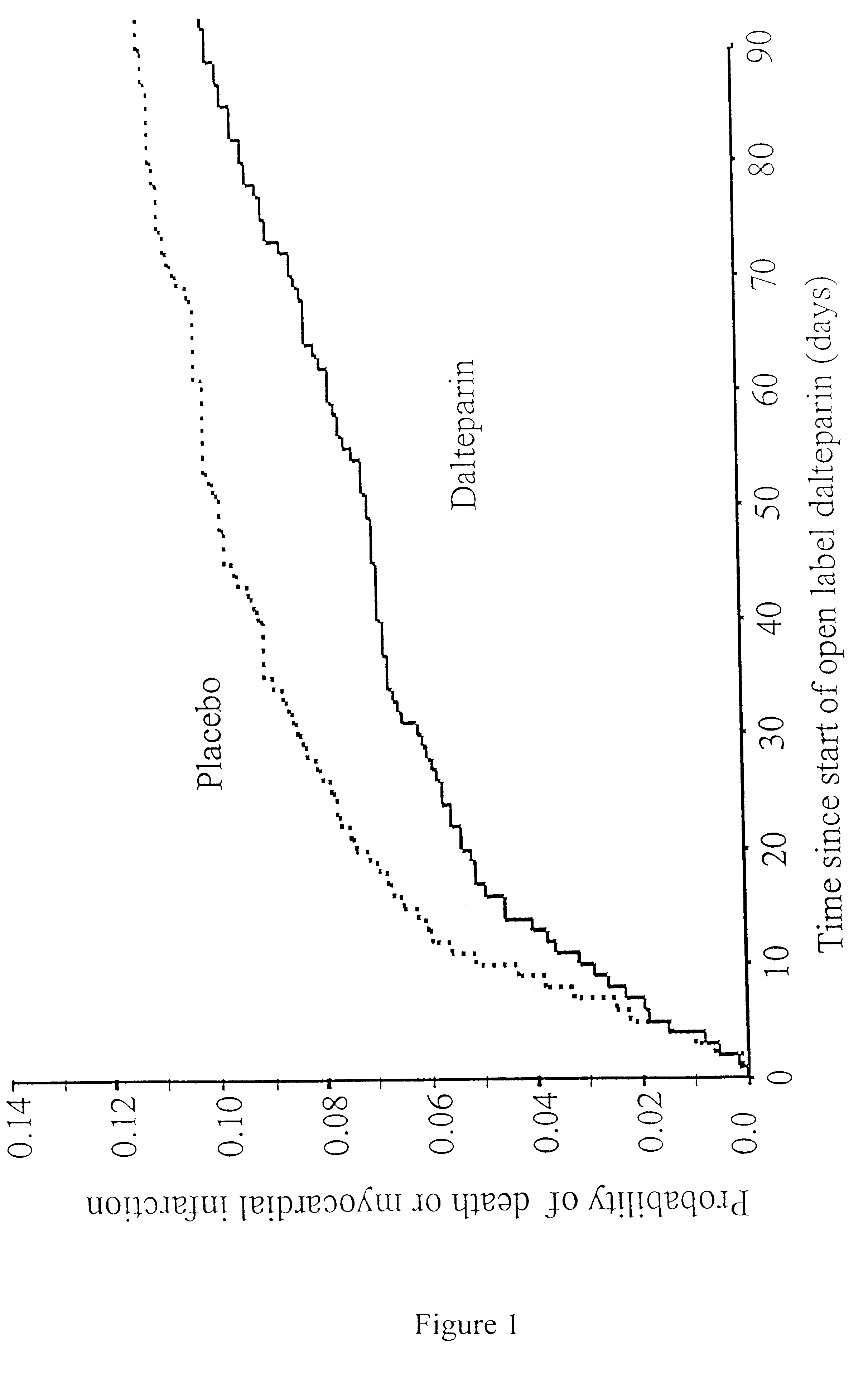
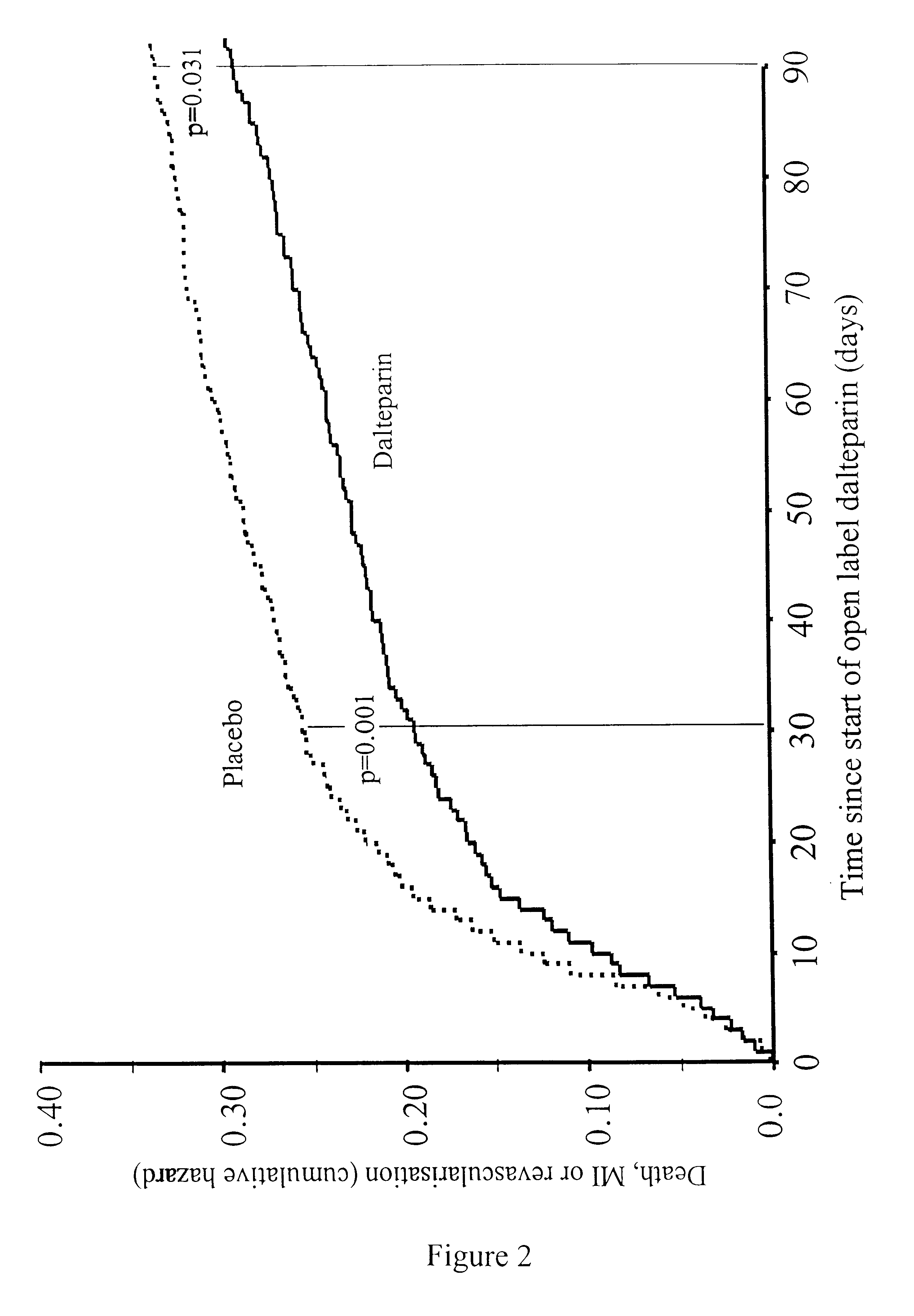
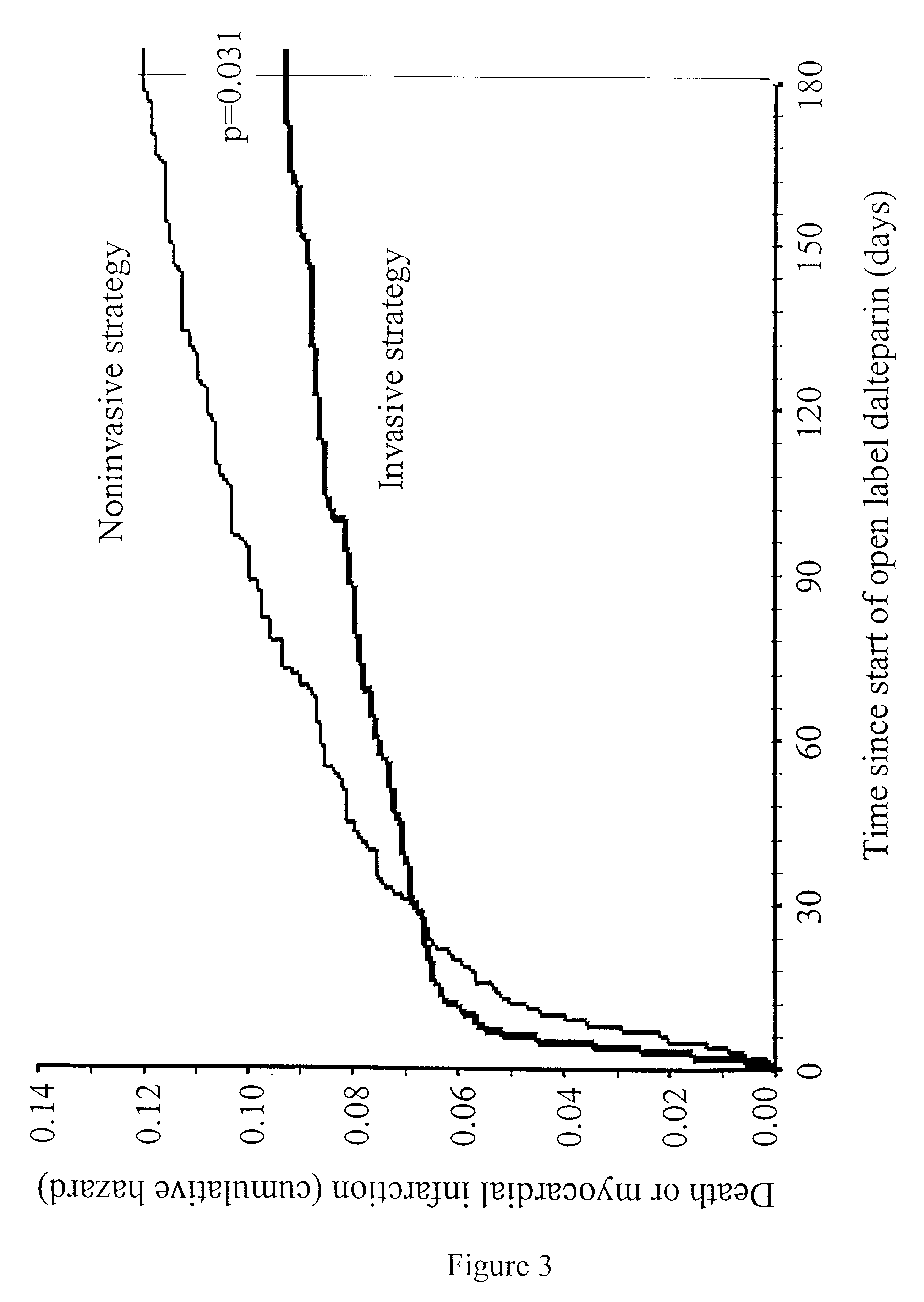
Method for treatment of unstable coronary artery disease by an early revascularisation together with administration of a low molecular weight heparin
InactiveUS6258798B1Reduce riskPromote resultsOrganic active ingredientsCardiovascular disorderDiseaseCoronary artery disease
The invention relates to a method for treatment of unstable coronary artery disease, which is characterised by an early revascularisation together with administration of a low molecular weight heparin. Preferably the low molecular weight heparin is administered up to revascularisation. The methods are also useful for treatment of unstable angina and not worsening chest pain and for treatment of non-Q-wave myocardial infarction (mild heart attack).
Owner:PHARMACIA AB
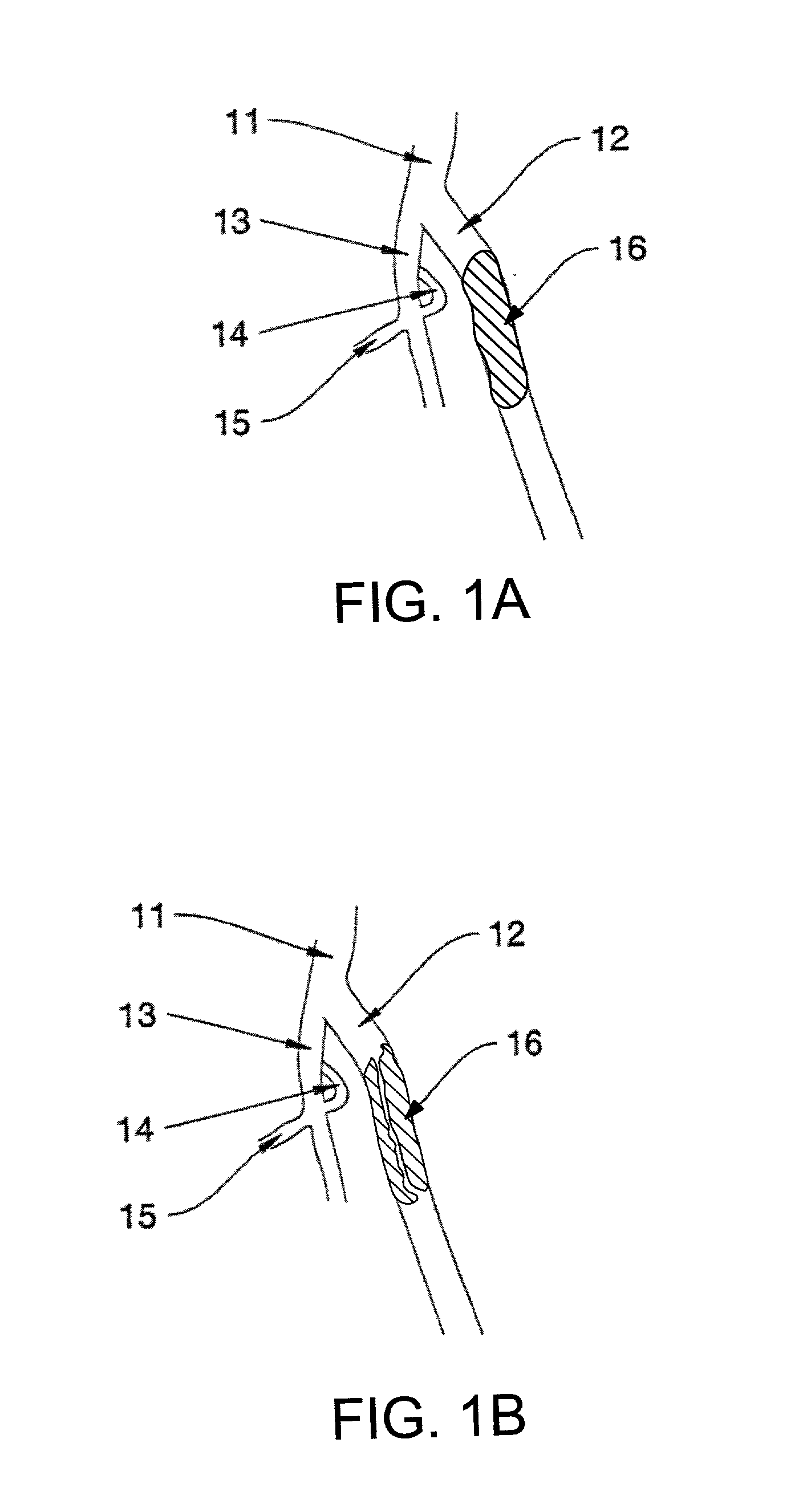
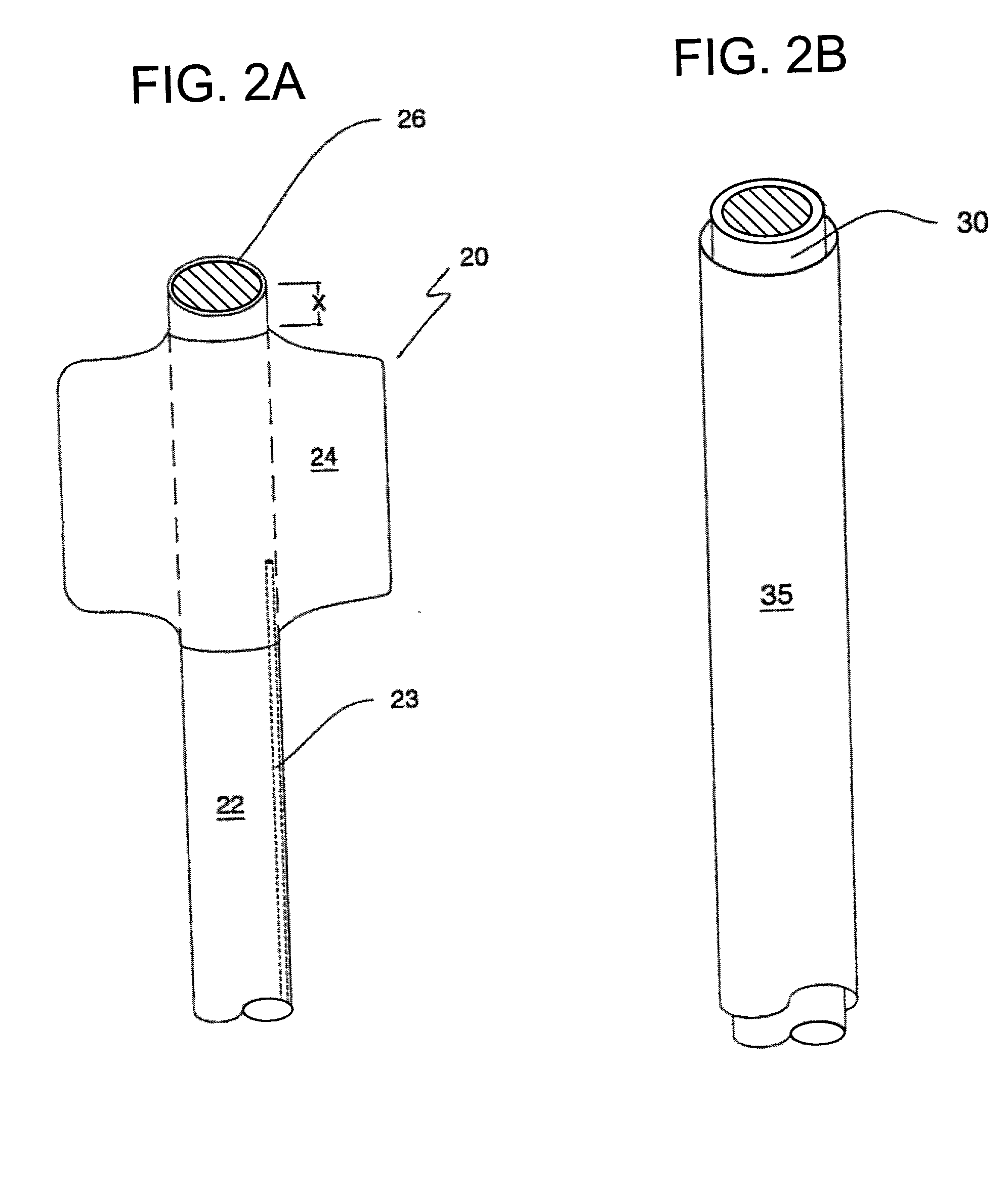
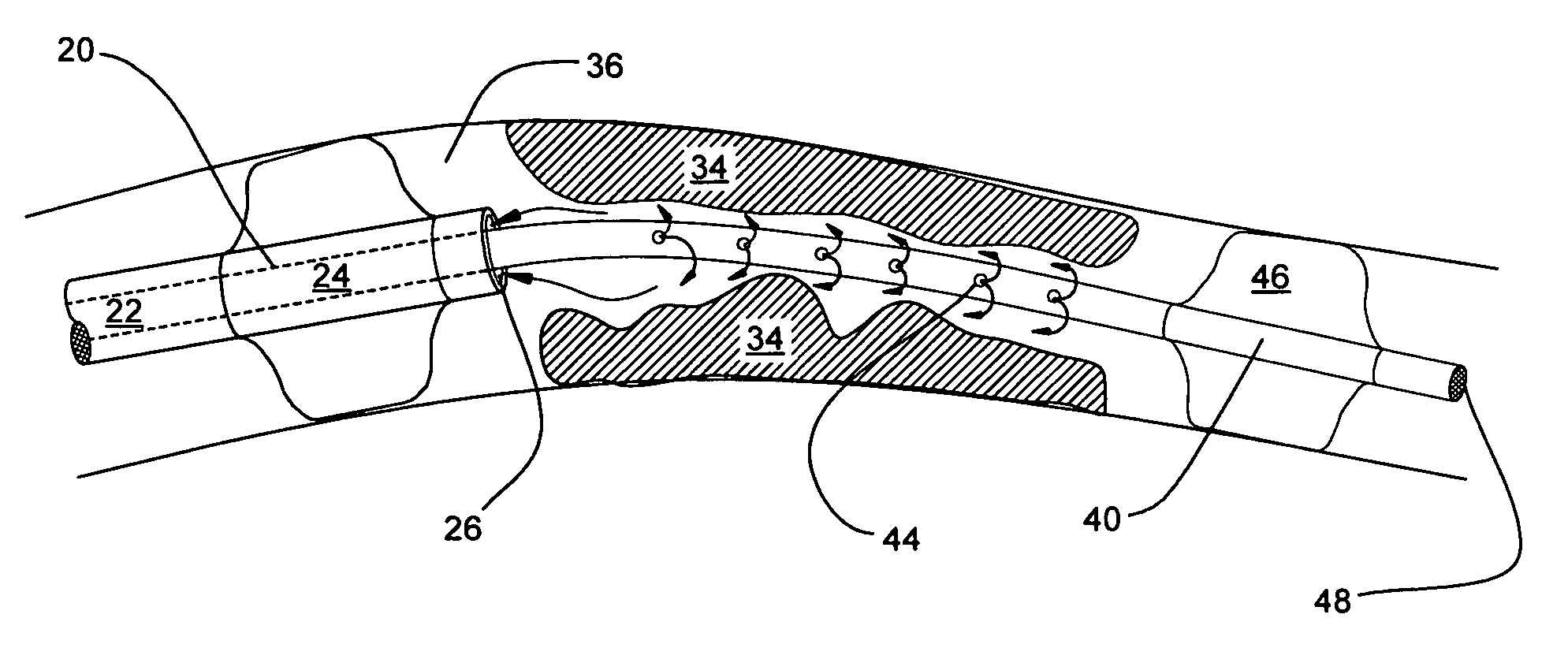
Catheter devices and methods for their use in the treatment of calcified vascular occlusions
Catheter devices and methods for their use in enhancing fluid flow through a vascular site occupied by a vascular occlusion are provided. The subject catheter devices include at least a first, second and third lumen, where: (a) the first lumen is used for delivery of an acidic dissolution solution to the vascular site; (b) the second lumen is used for delivery of a buffer solution to the vascular site; and (c) the third lumen is used for removal of fluid from the vascular site. In many preferred embodiments, the first, second and third lumens are coaxial. In practicing the subject methods, the vascular site is flushed simultaneously with an acidic dissolution fluid and a buffer solution, where flushing is carried out in a manner such that only a surface of the vascular occlusion is contacted with the acidic dissolution fluid and the remainder of the vascular site is not contacted with fluid having a pH that is lower than about 4. Flushing is carried out in this manner for a period of time sufficient for fluid flow through the vascular site to be enhanced, e.g. increased or established. The subject catheter devices and methods find use in the treatment of a variety of different vascular diseases characterized by the presence of calcified vascular occlusions, including peripheral and coronary vascular diseases.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
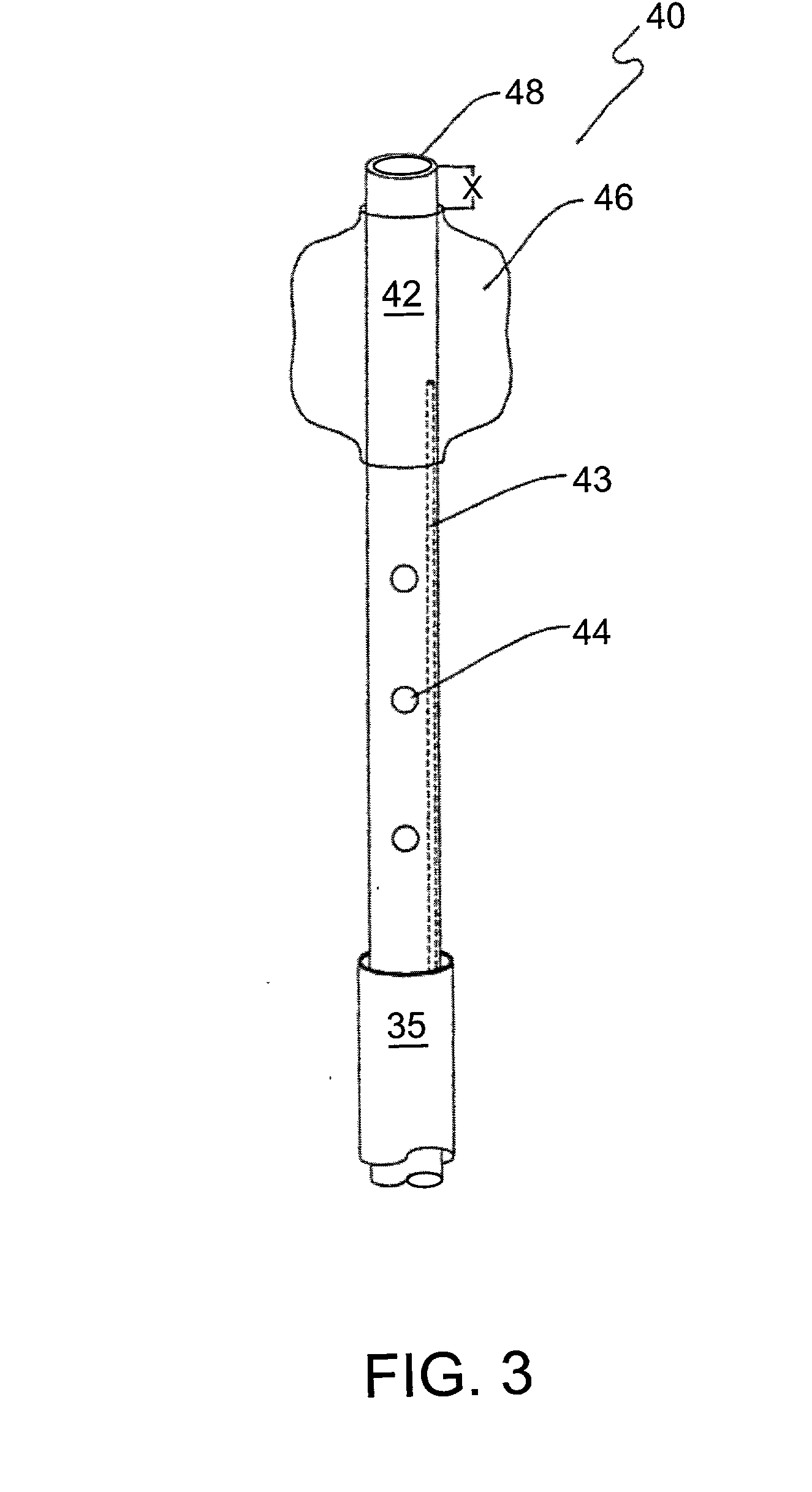
Catheter systems and methods for their use in the treatment of calcified vascular occlusions
ActiveUS20060161103A1Improve fluid flowBalloon catheterMulti-lumen catheterCoronary circulationCoronary vascular disease
Catheter systems and methods for their use in enhancing fluid flow through a vascular site occupied by a vascular occlusion are provided. The subject catheter systems include at least an aspiration catheter and at least one of a total occlusion insert catheter and a partial occlusion insert catheter, where the insert catheters are capable of being slidably moved in the lumen of the aspiration catheter. In practicing the subject methods, a surface of the vascular occlusion is flushed with an acidic dissolution fluid using the subject catheter systems for a period of time sufficient for fluid flow through the vascular site to be enhanced, e.g. increased or established. The subject catheter systems and methods find use in the treatment of a variety of different vascular diseases characterized by the presence of calcified vascular occlusions, including peripheral and coronary vascular diseases.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Novel heterocyclic analogs of diphenylethylene compounds
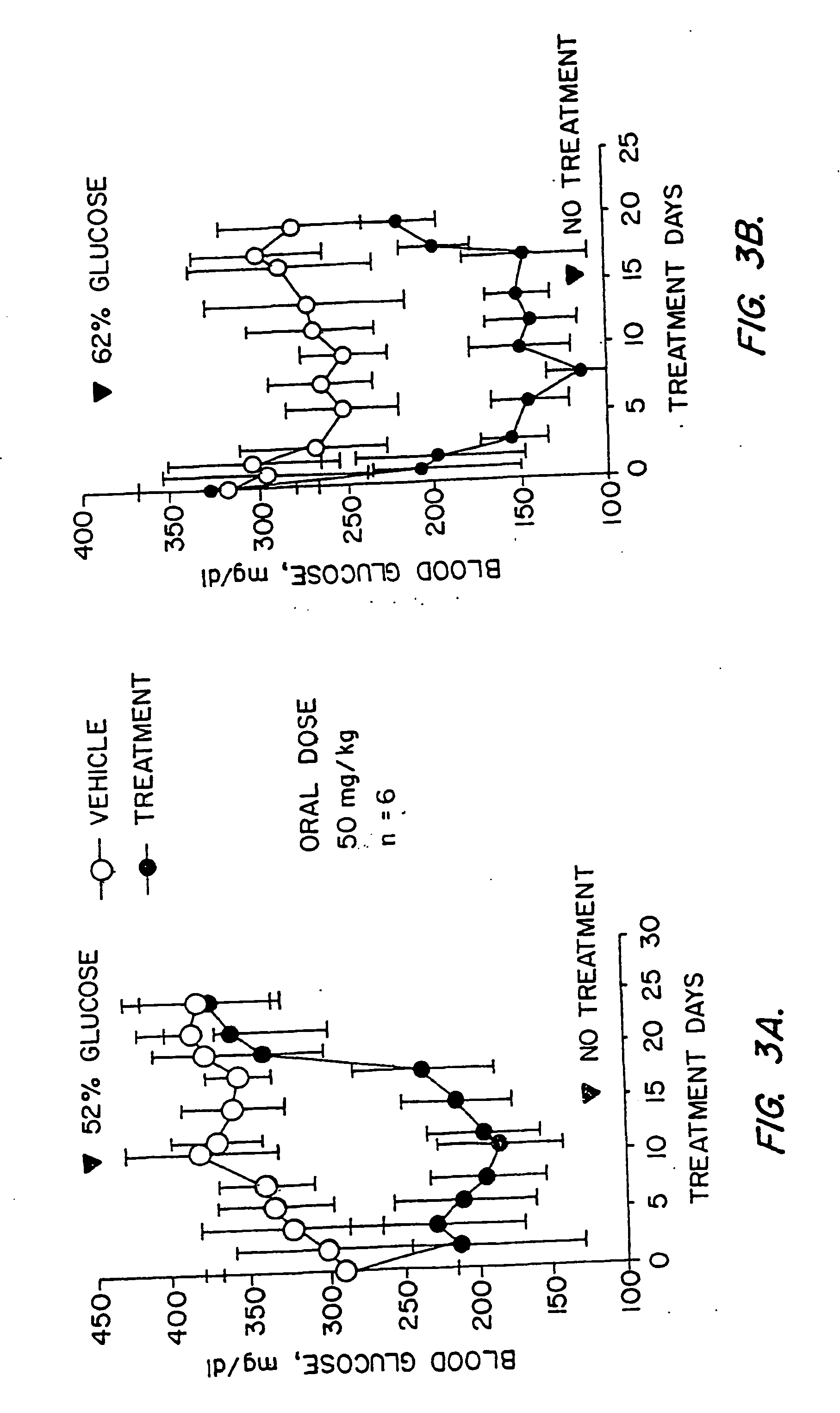
Novel diphenylethylene compounds and derivatives thereof containing thiazolidinedione or oxazolidinedione moieties are provided which are effective in lowering blood glucose level, serum insulin, triglyceride and free fatty acid levels in animal models of Type II diabetes. The compounds are disclosed as useful for a variety of treatments including the treatment of inflammation, inflammatory and immunological diseases, insulin resistance, hyperlipidemia, coronary artery disease, cancer and multiple sclerosis.
Owner:THERAKOS INC
Compositions and methods related to heart failure
InactiveUS20060014828A1Reduce in quantityShorten the construction periodBiocideNervous disorderDiseaseType B Natriuretic Peptide
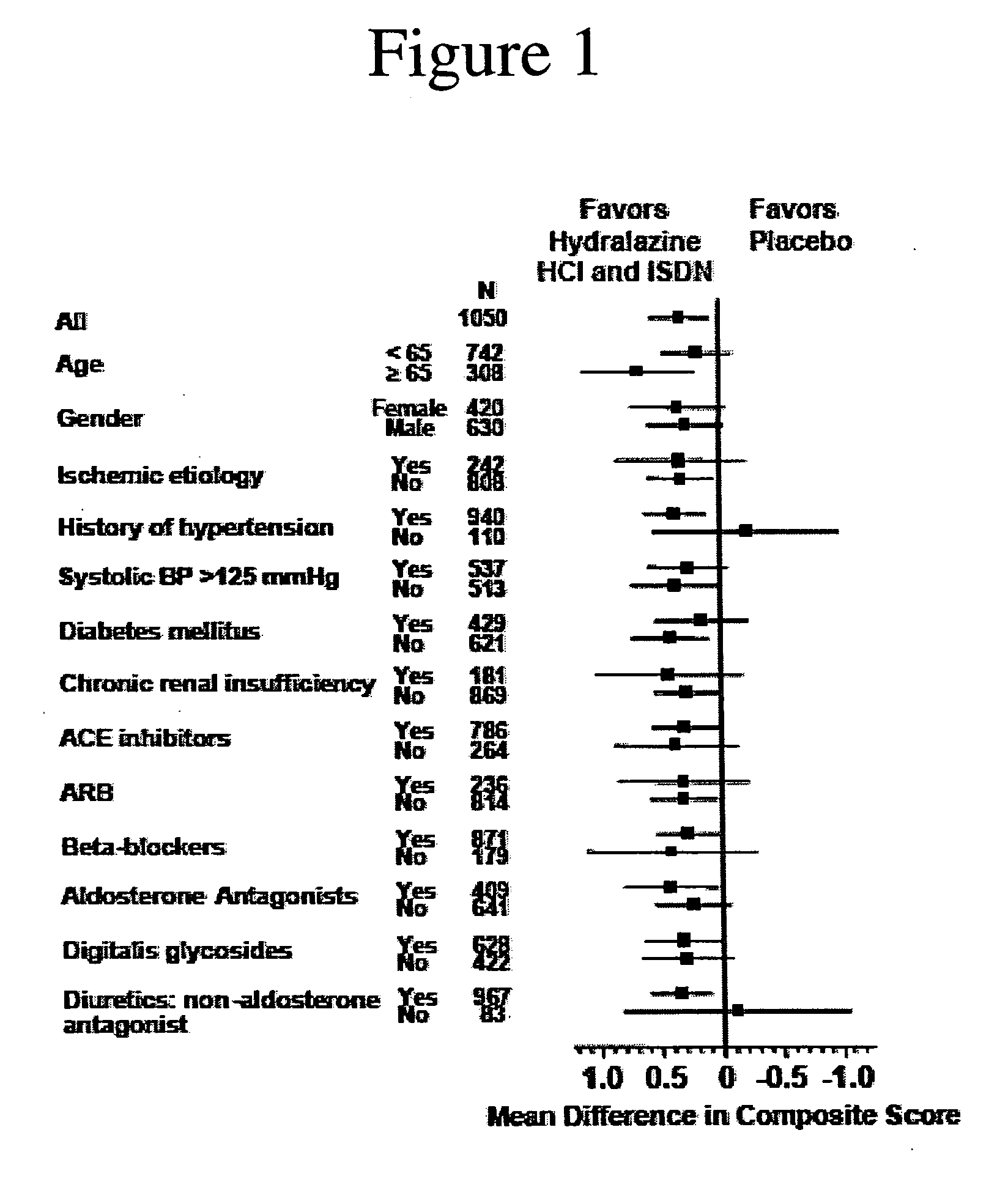
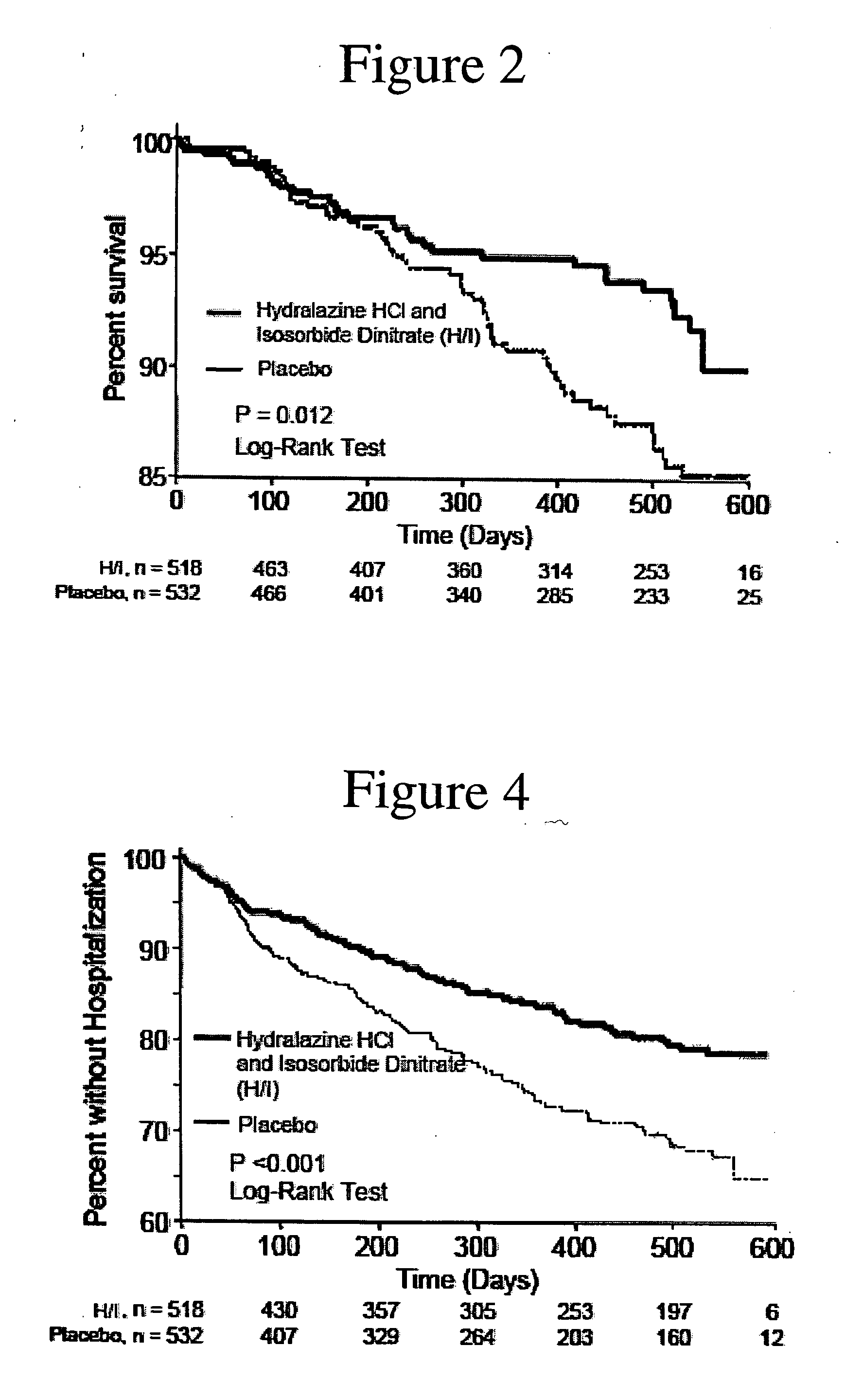
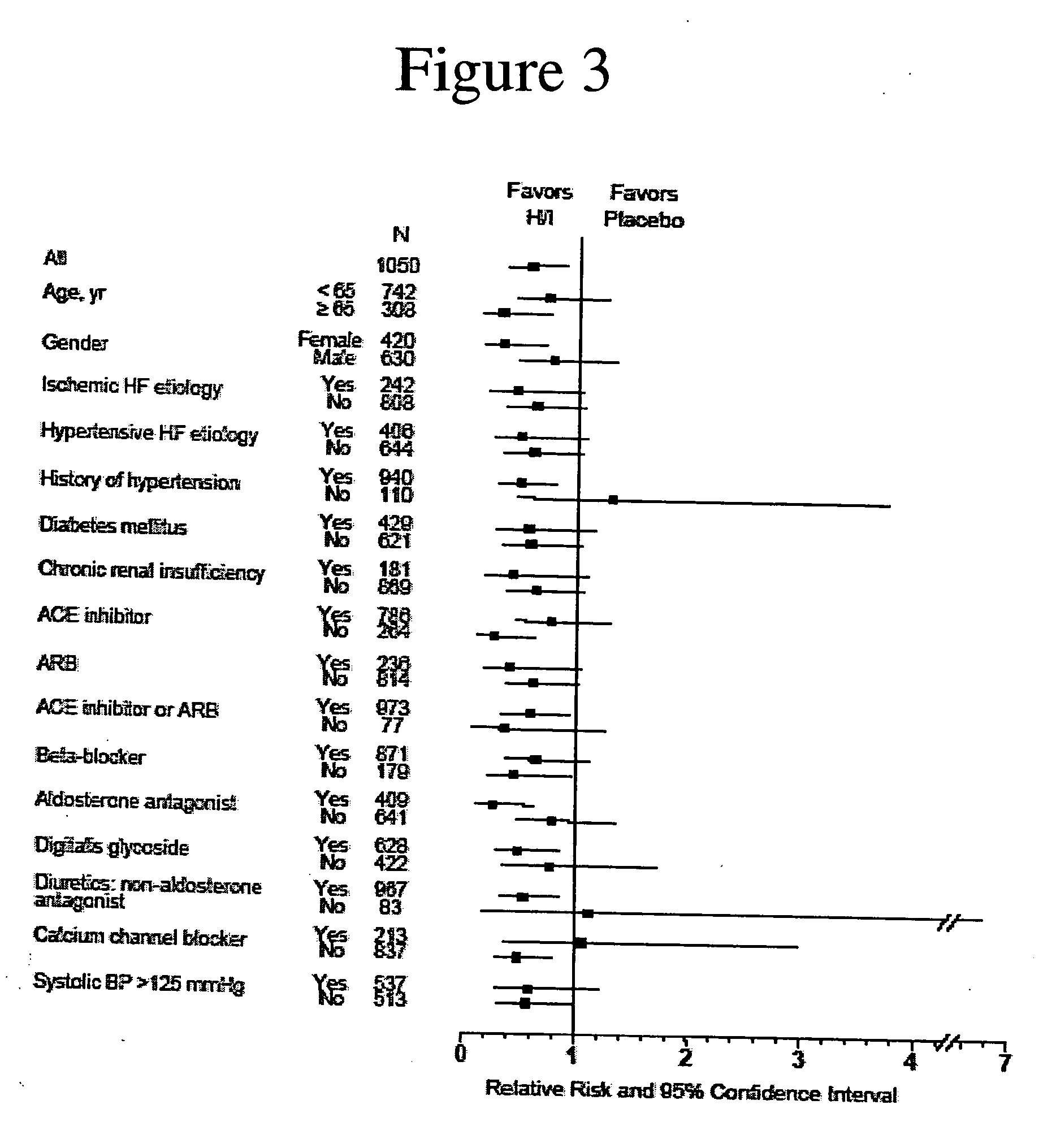
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays (i.e., two or more hospital stays); (e) reducing the number of hospital admissions for heart failure; (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits); (g) increasing the left ventricular ejection fraction in a heart failure patient; (h) treating a sexual dysfunction (e.g., erectile dysfunction and female sexual dysfunction) (j) treating a headache in a heart failure patient by administering a non-steroidal antiinflammatory compound (i.e., NSAIDs); (k) treating a heart failure patient who has a history of hypertension (but who is not currently diagnosed with hypertension); (l) improving the quality of life in a heart failure patient based on the Minnesota Living with heart failure questionnaire; (m) decreasing the levels of B-type natriuretic peptide; (n) treating hypertension in a heart failure patient; (o) lowering blood pressure in a heart failure patient; (p) treating labile hypertension; (q) treating idiopathic hypertension; (r) increasing patient compliance with medication dosing in a heart failure patient; (s) treating hypertension in a patient with a dilated heart; (t) treating ischemic disease and / or coronary artery disease; and (u) reducing cardiomegaly in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Methods for Disease Therapy
InactiveUS20100130526A1Improve isolationHigh throughput analysisBiocideBioreactor/fermenter combinationsDisease phenotypeCoronary artery disease
The present invention discloses disease-linked SNPs, microRNAs, and microRNA-targeted mRNAs relevant to the pathogenesis of several major human disorders including, but not limited to, multiple types of cancers, type 2 diabetes, type 1 diabetes, Crohn's disease, coronary artery disease, hypertension, rheumatoid arthritis, bipolar disorder. Also provided are methods for the identification of disease phenotype-defining sets of SNPs, microRNAs, and mRNAs that are defined here as a “consensus disease phenocode” as well as methods of using the information provided by these consensus disease phenocodes for various diagnostic, prognostic, and / or therapeutic applications.
Owner:ORDWAY RES INST
Biomarkers for coronary artery disease
ActiveCN107075563AGuaranteed complianceGuaranteed accuracyMicrobiological testing/measurementCoronary artery diseaseCoronary heart disease
Owner:BGI SHENZHEN CO LTD +1
Methods of diagnosing cardiovascular disease
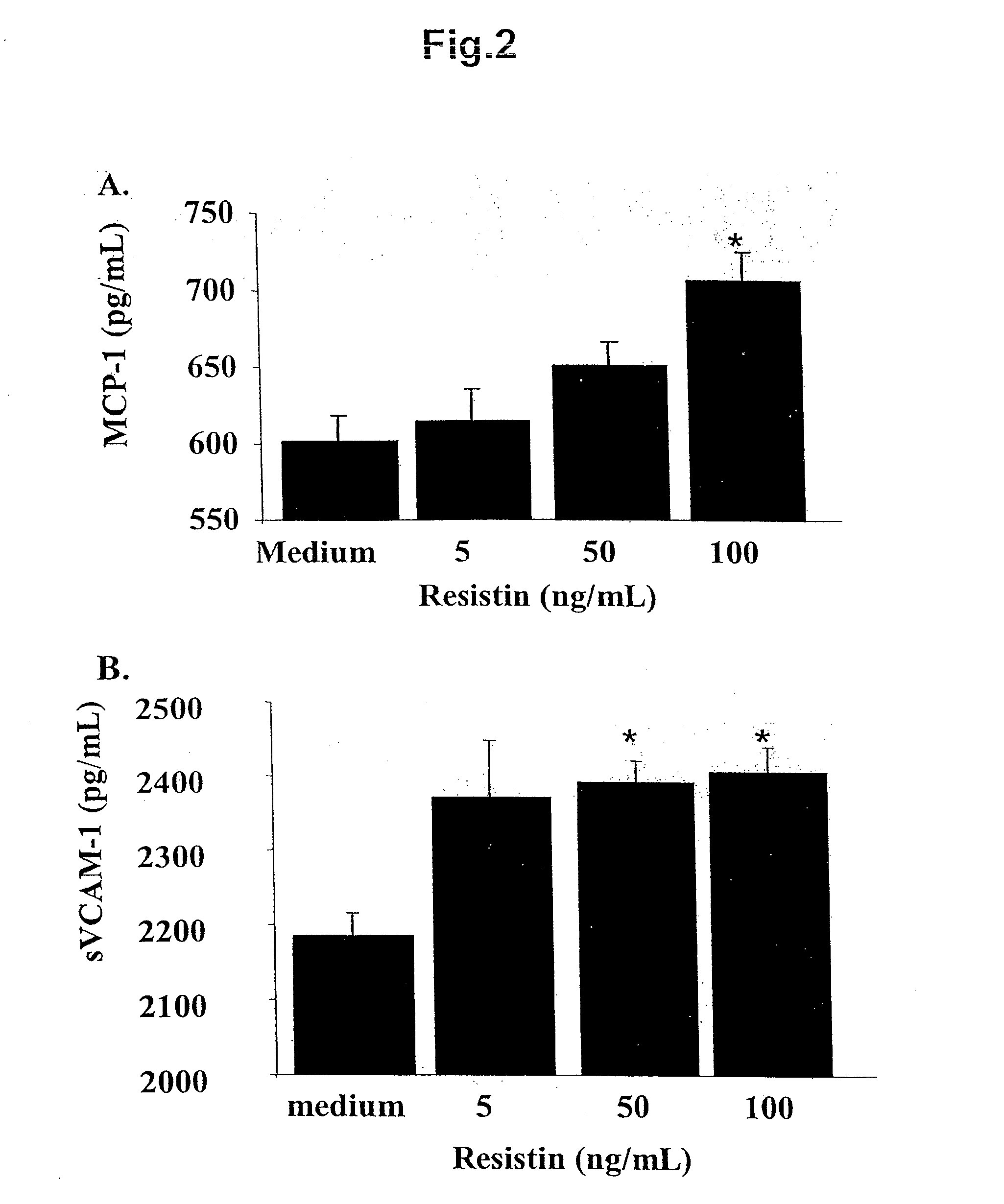
InactiveUS20100105046A1Assessing the prognosis of vascular conditions and/or vascular eventsReduce severityMicrobiological testing/measurementDisease diagnosisCvd riskRisk stroke
The invention relates to predicting which individuals are at risk of developing atherosclerotic vascular disease, and once having disease, which individuals are at risk of experiencing plaque rupture which, depending on the site of the plaque, could produce myocardial infarction, stroke, critical limb ischemia, or other vascular event. The invention further relates to methods of diagnosing and aiding in the diagnosis of vascular conditions such as atherosclerosis, premature coronary artery disease and coronary artery disease, by detecting a resistin gene product in an individual. The invention further relates to methods of predicting, and aiding in predicting, the likelihood that an individual will experience a vascular event, such as but not limited to, a myocardial infarction, acute coronary syndrome, stroke, transient ischemic attack (TIA), or critical limb ischemia.
Owner:MEDSTAR HEATH INC
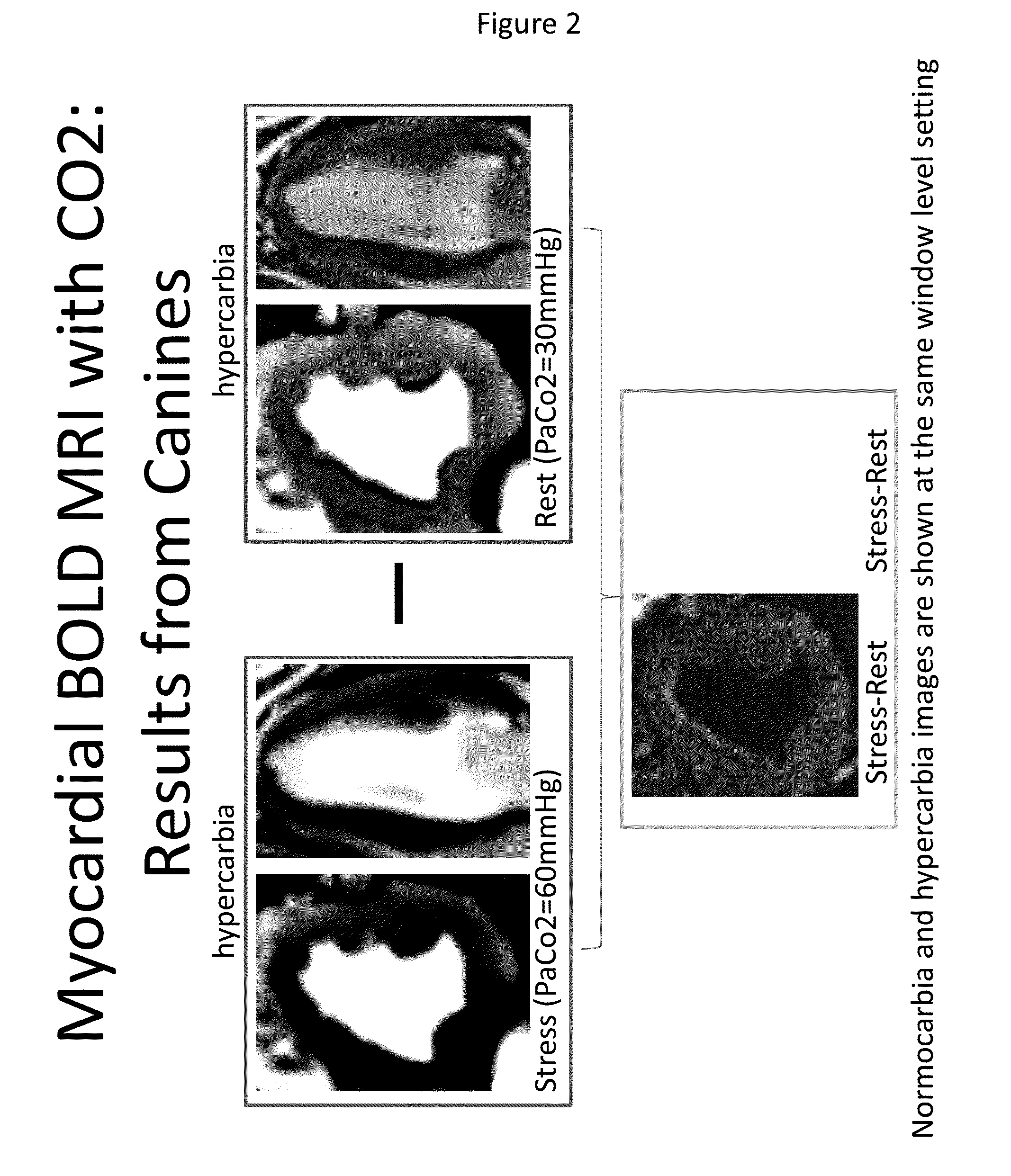
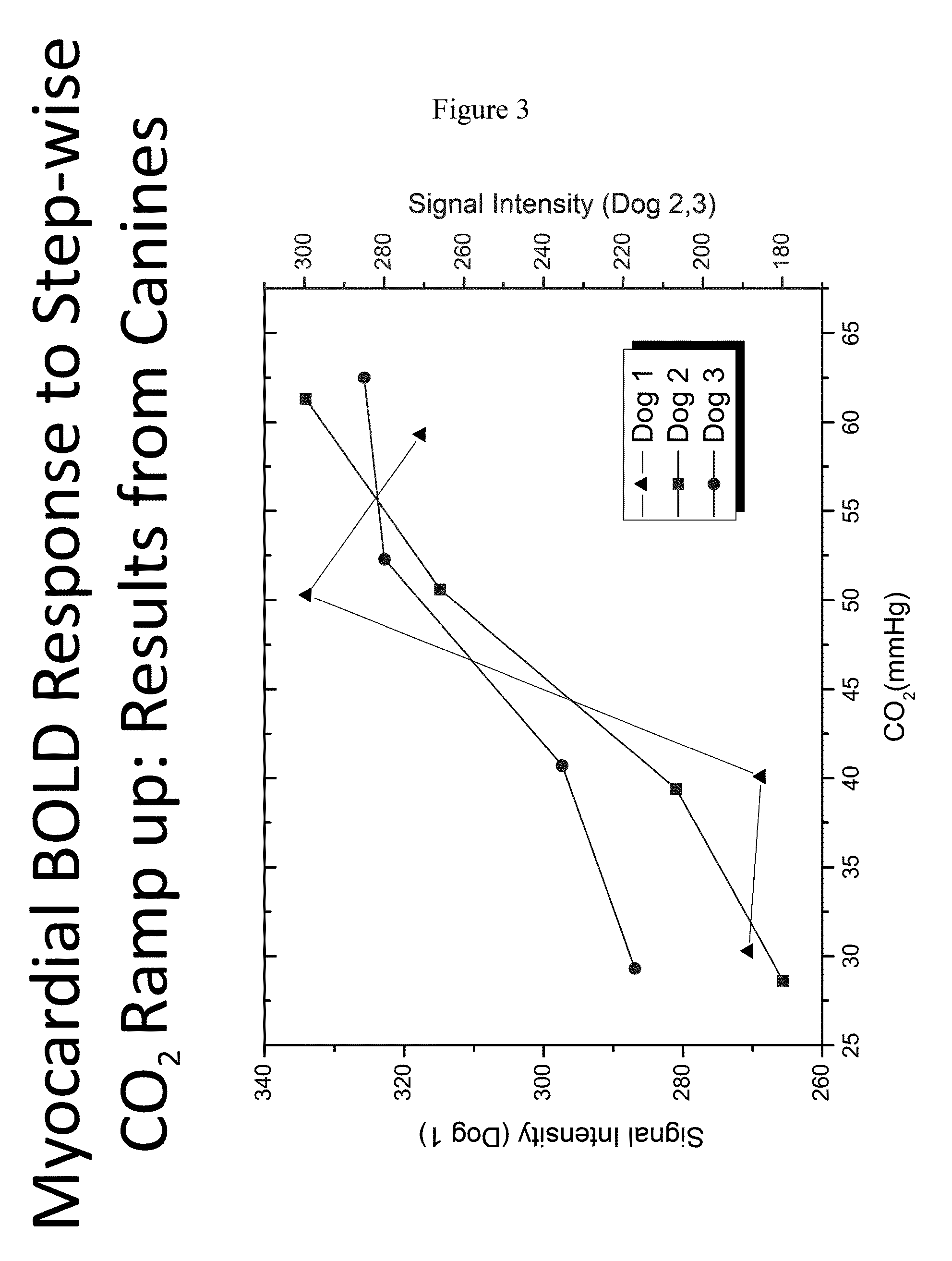
Assessment of coronary heart disease with carbon dioxide
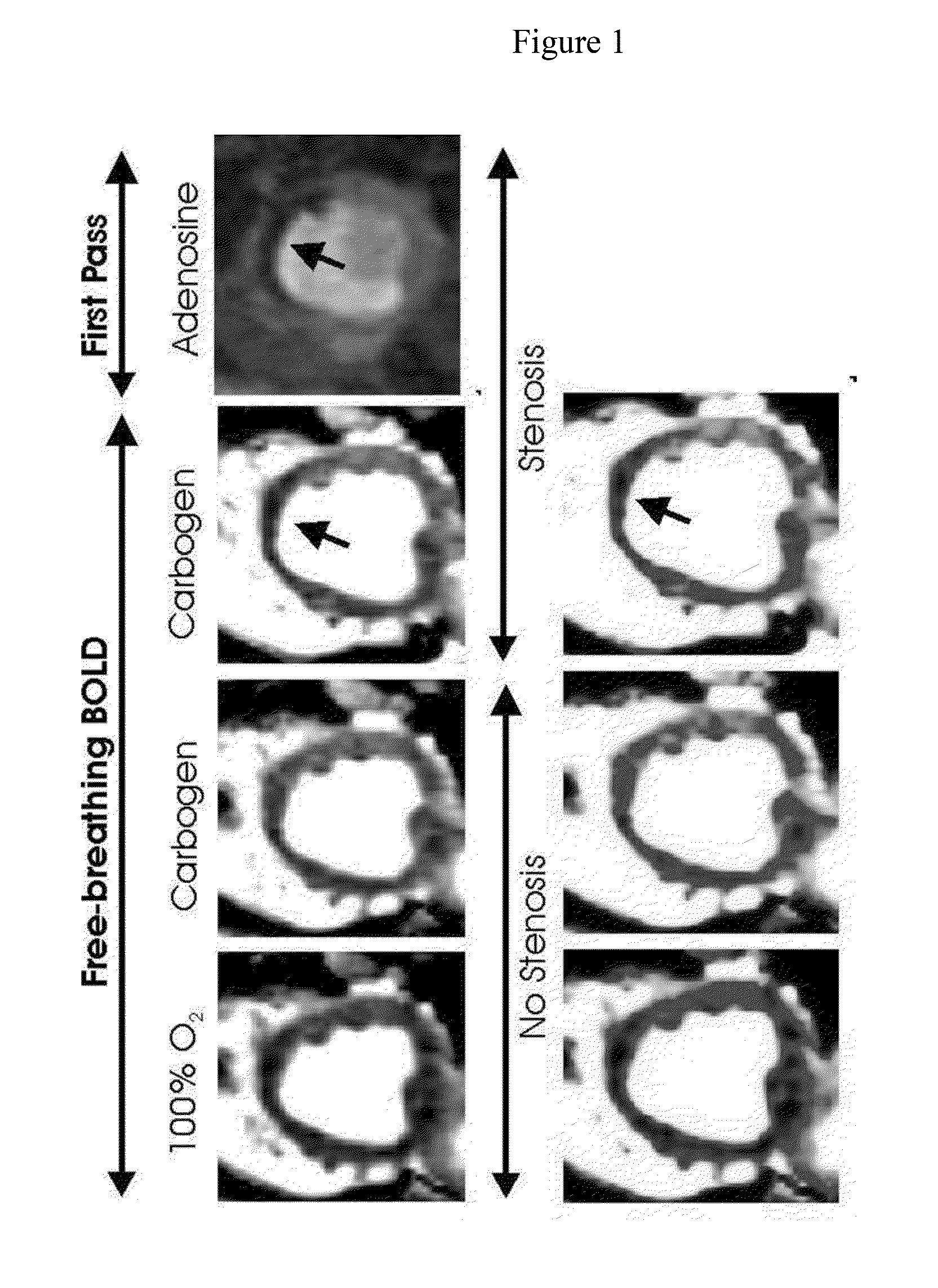
ActiveUS20140088406A1Induce hyperemiaHighly cost-effectiveStethoscopeCatheterControl subjectsCoronary artery disease
The invention provides methods for diagnosing coronary heart disease in a subject in need thereof comprising administering an admixture comprising CO2 to a subject to reach a predetermined PaCO2 in the subject to induce hyperemia, monitoring vascular reactivity in the subject and diagnosing the presence or absence of coronary heart disease in the subject, wherein decreased vascular reactivity in the subject compared to a control subject is indicative of coronary heart disease. The invention also provides methods for increasing sensitivity and specificity of BOLD MRI.
Owner:CEDARS SINAI MEDICAL CENT
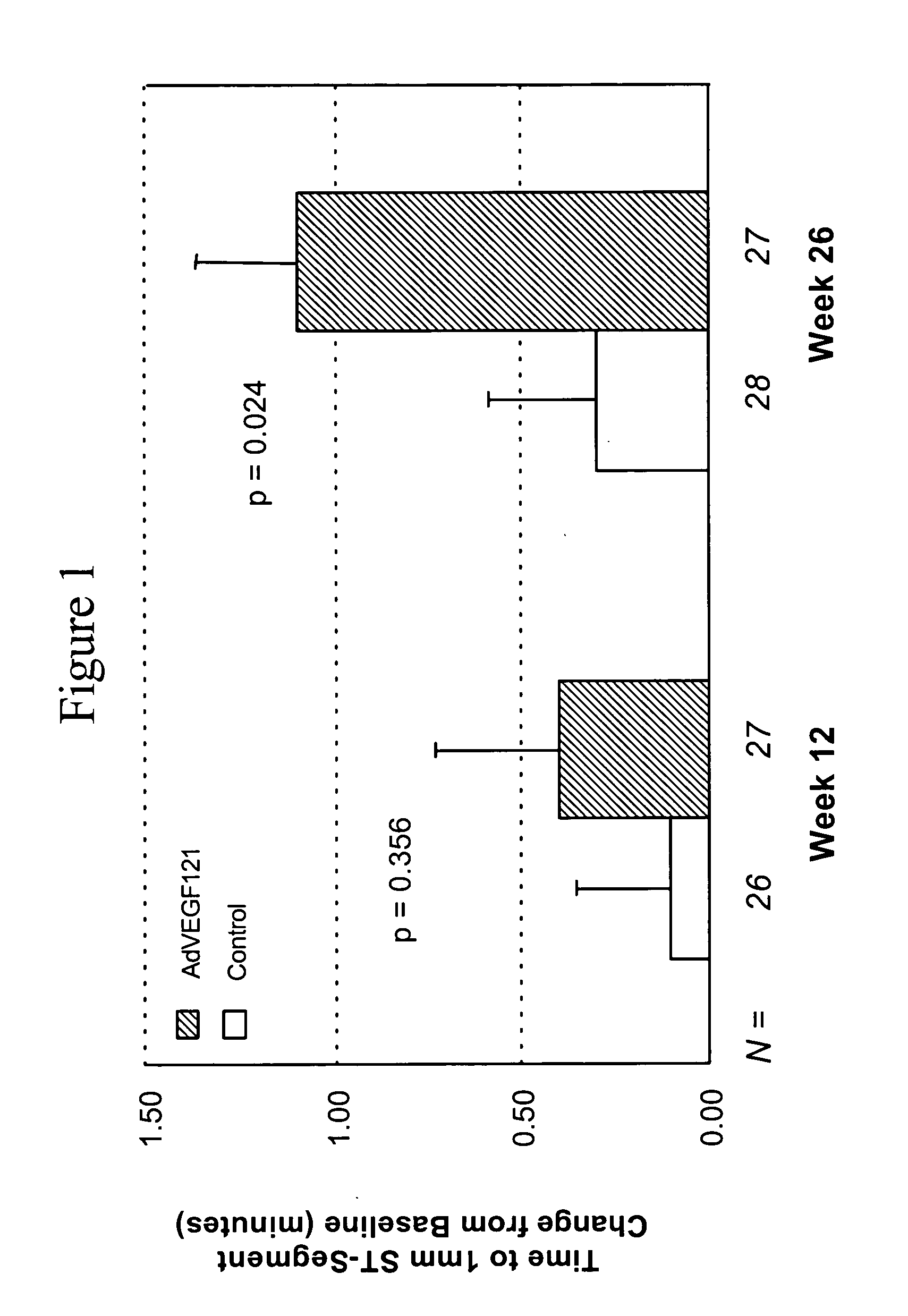
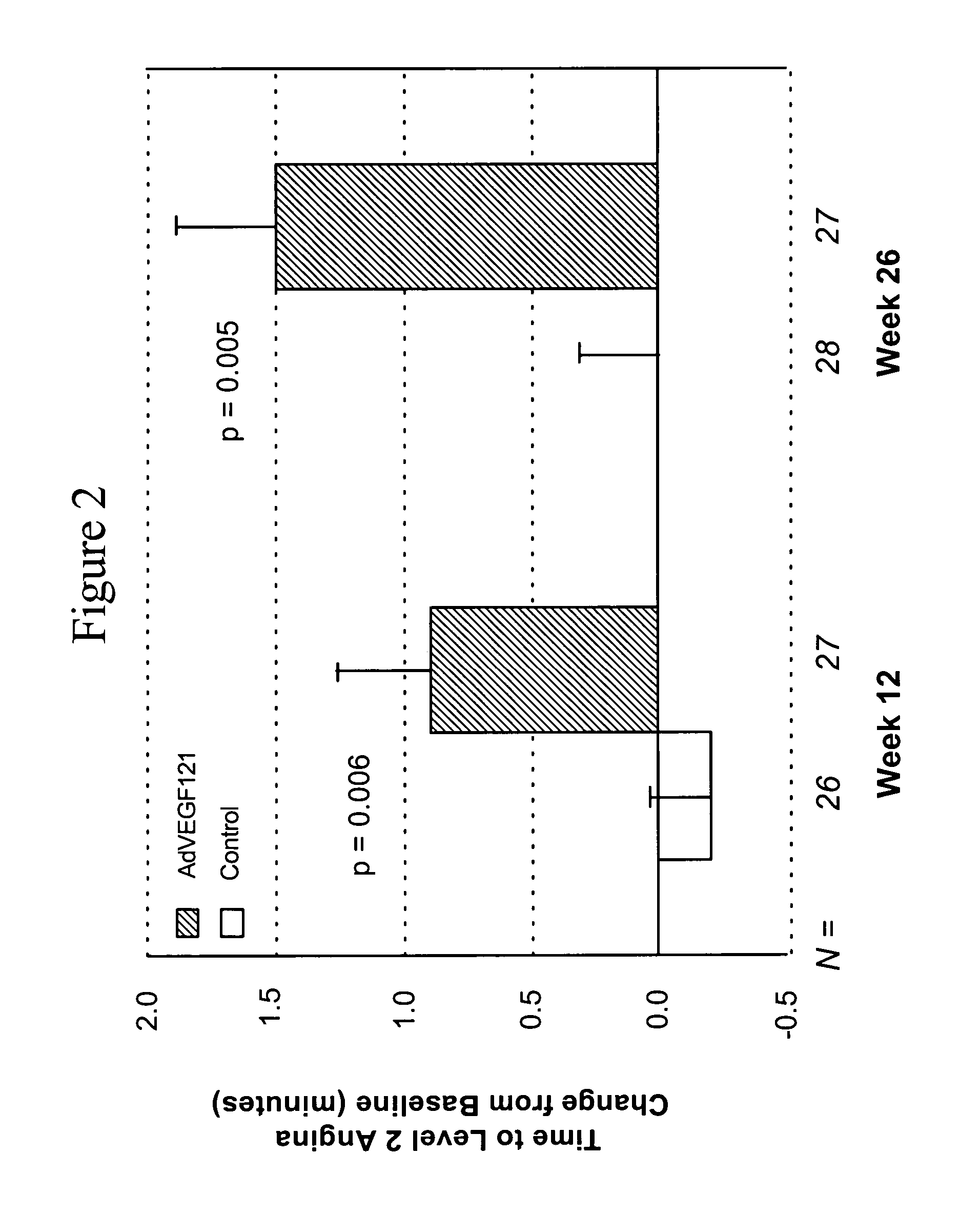
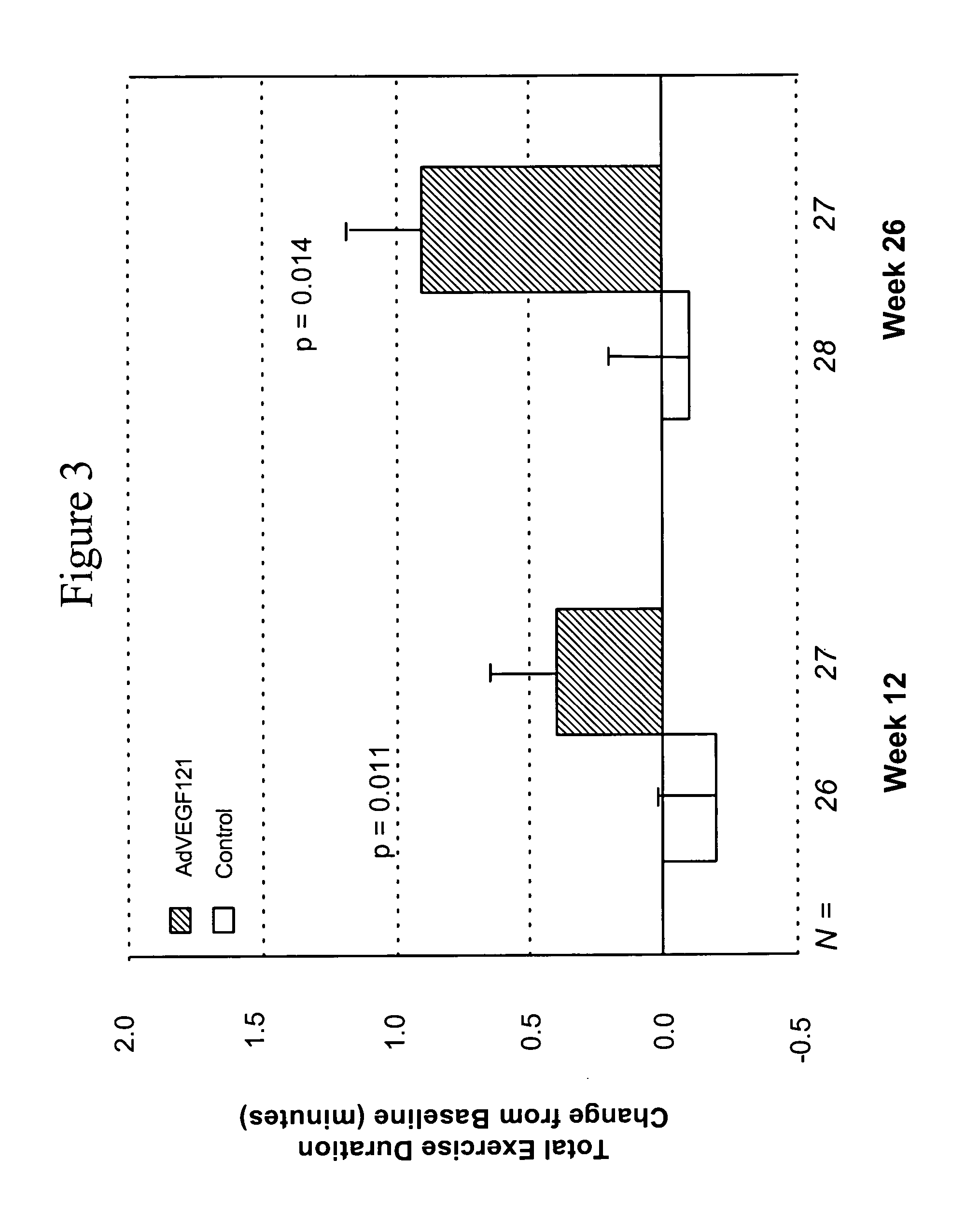
Coronary artery disease treatment
The invention provides a method of treating coronary artery disease in a human patient comprising directly injecting into an ischemic cardiac muscle, via multiple injections to different points of the cardiac muscle, a dose of a pharmaceutical composition comprising a pharmaceutically acceptable carrier and a replication-deficient adenoviral vector comprising a nucleic acid sequence encoding an angiogenic peptide operably linked to a promoter, whereby the coronary artery disease is treated.
Owner:WARNER-LAMBERT CO +1
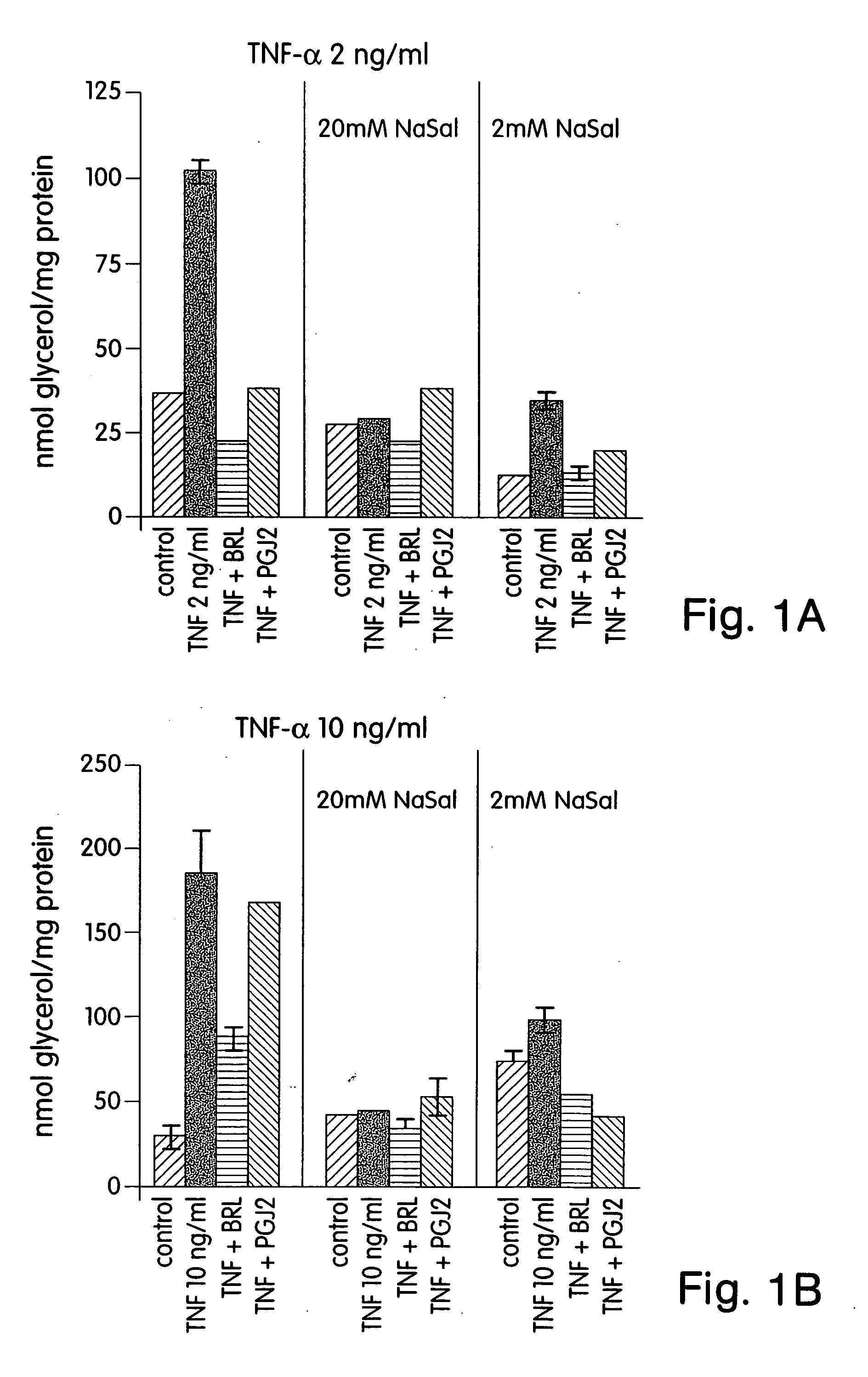
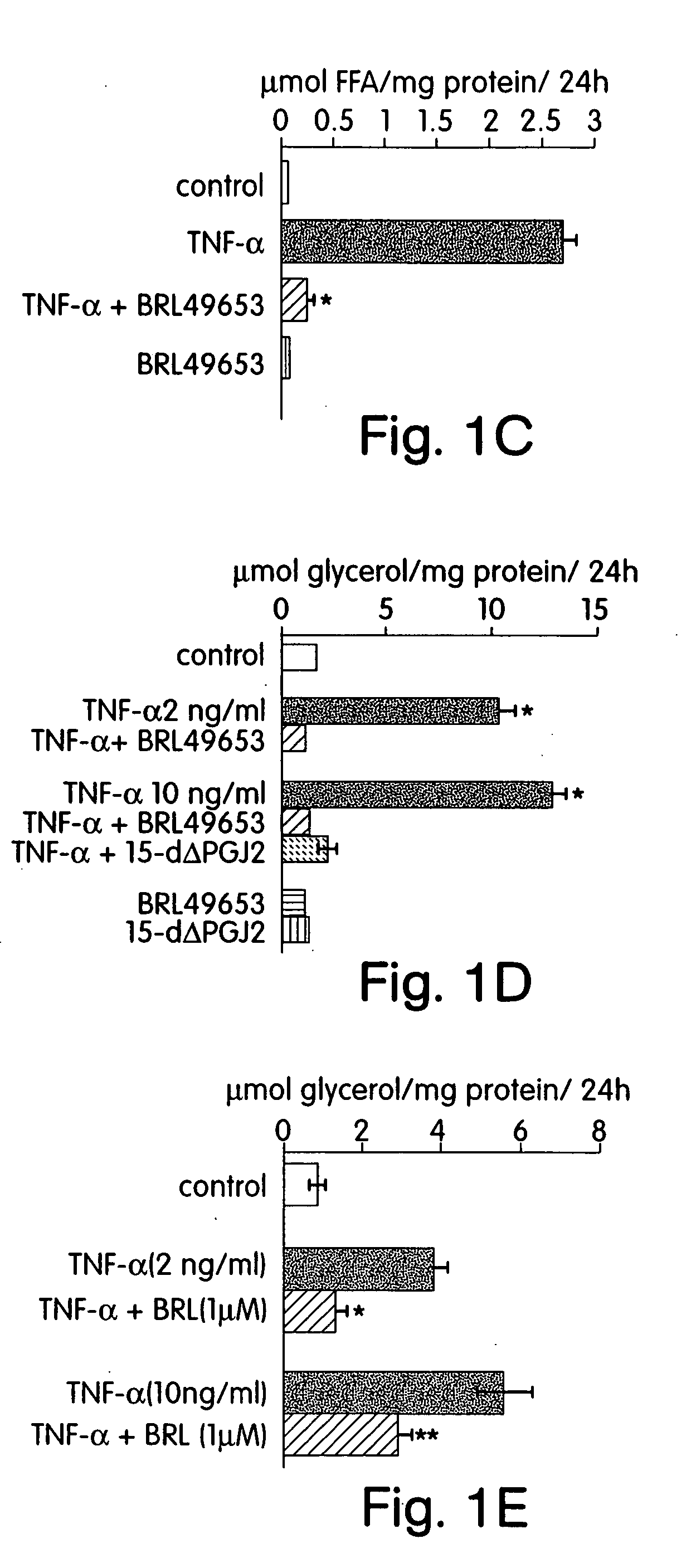
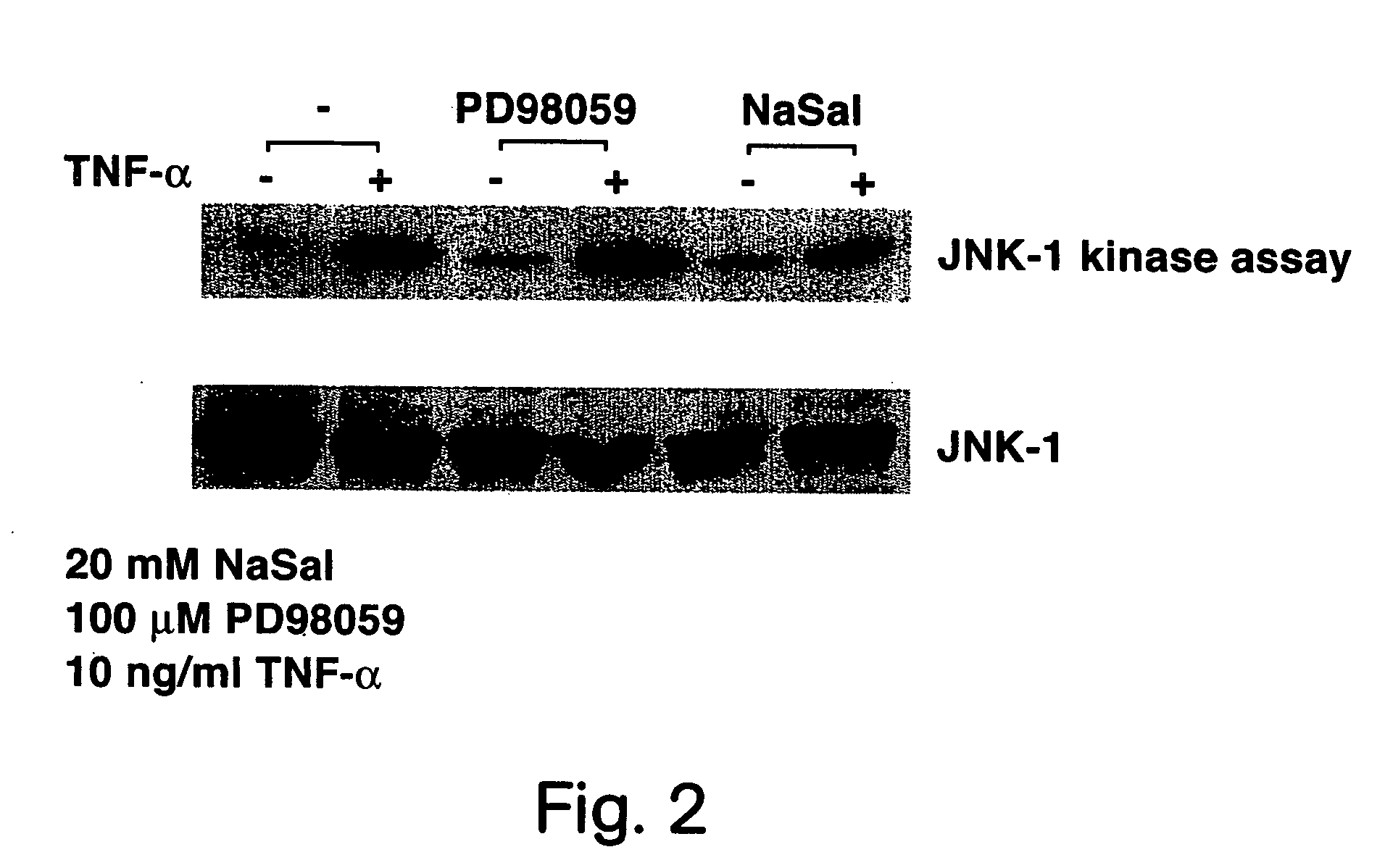
Methods for treating and preventing insulin resistance and related disorders
The invention provides methods, therapeutics and kits for treating and preventing diseases or conditions associated with excessive lipolysis, in particular TNF-α induced lipolysis, and / or excessive free fatty acid levels. Exemplary conditions include insulin-resistance, diabetes, in particular NIDDM, obesity, glucose intolerance, hyperinsulinemia, polycystic ovary syndrome, and coronary artery disease. In a preferred embodiment, the method includes administering to a subject in need a pharmaceutically effective amount of an inhibitor of the JNK signal transduction pathway and / or an inhibitor of the MAPK / ERK signal transduction pathway.
Owner:GREENBERG ANDREW S
Detection of a predisposition for the development of coronary artery disease
InactiveUS20050053956A1Increased riskMicrobiological testing/measurementFermentationDiseaseCoronary arteries
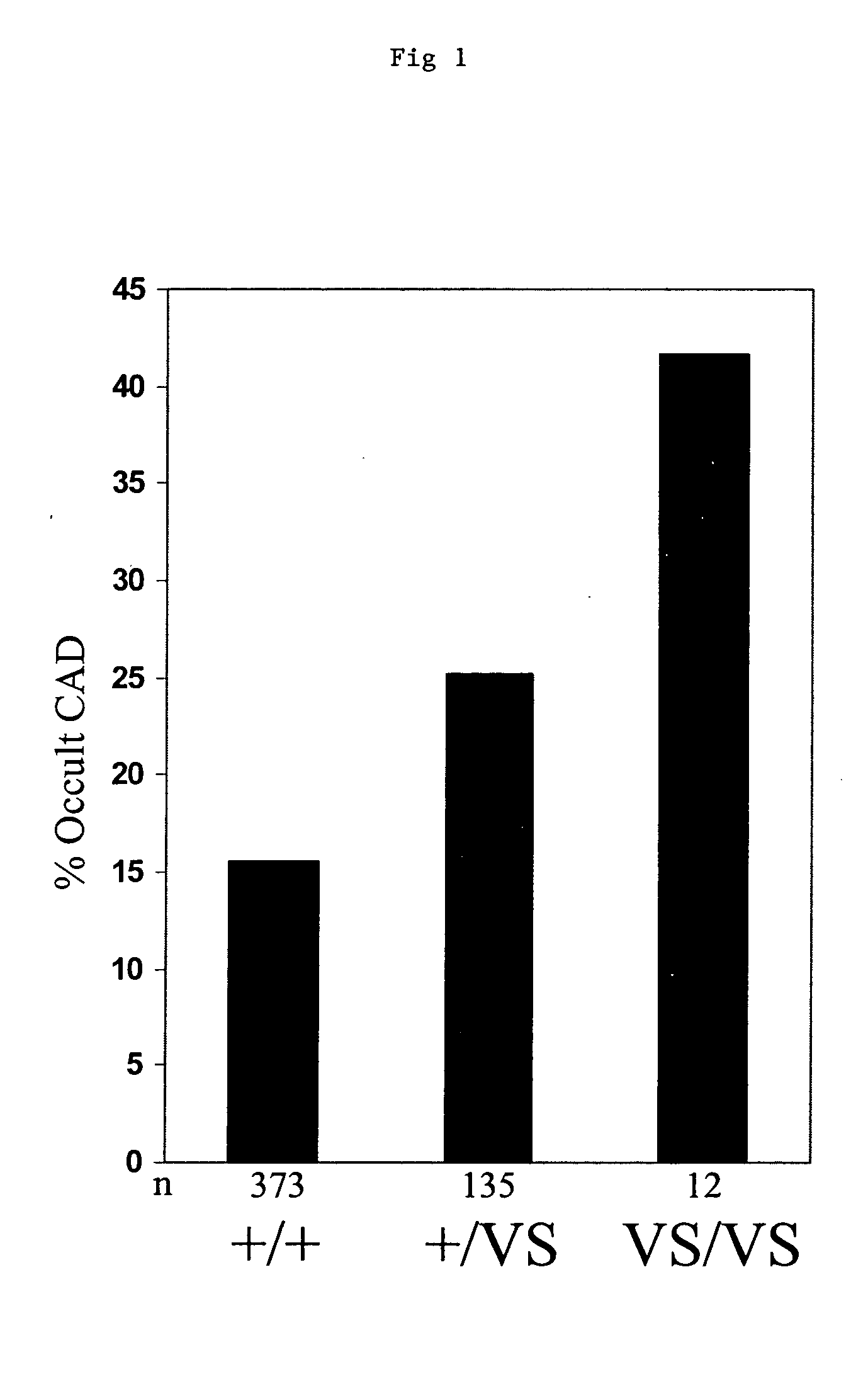
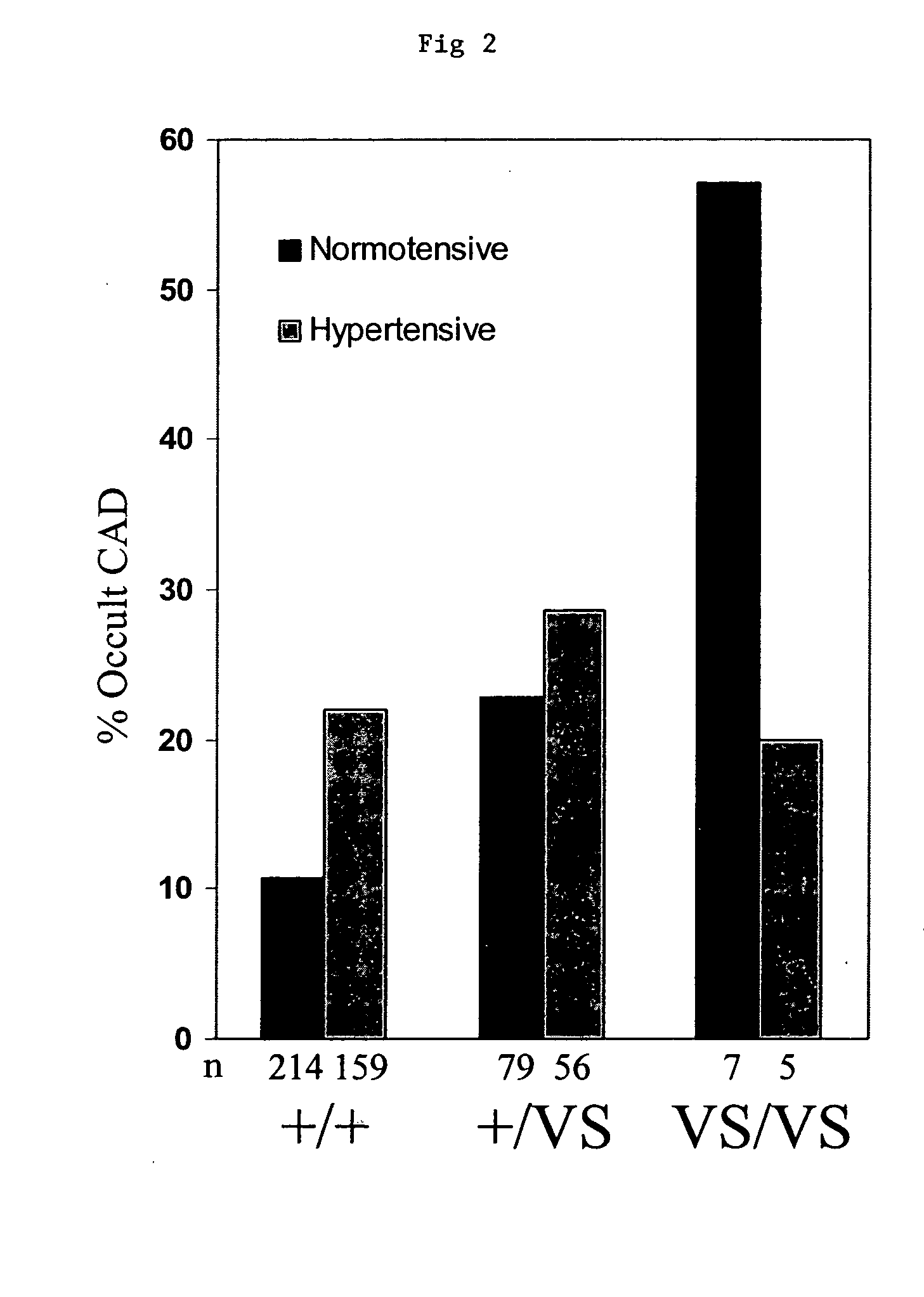
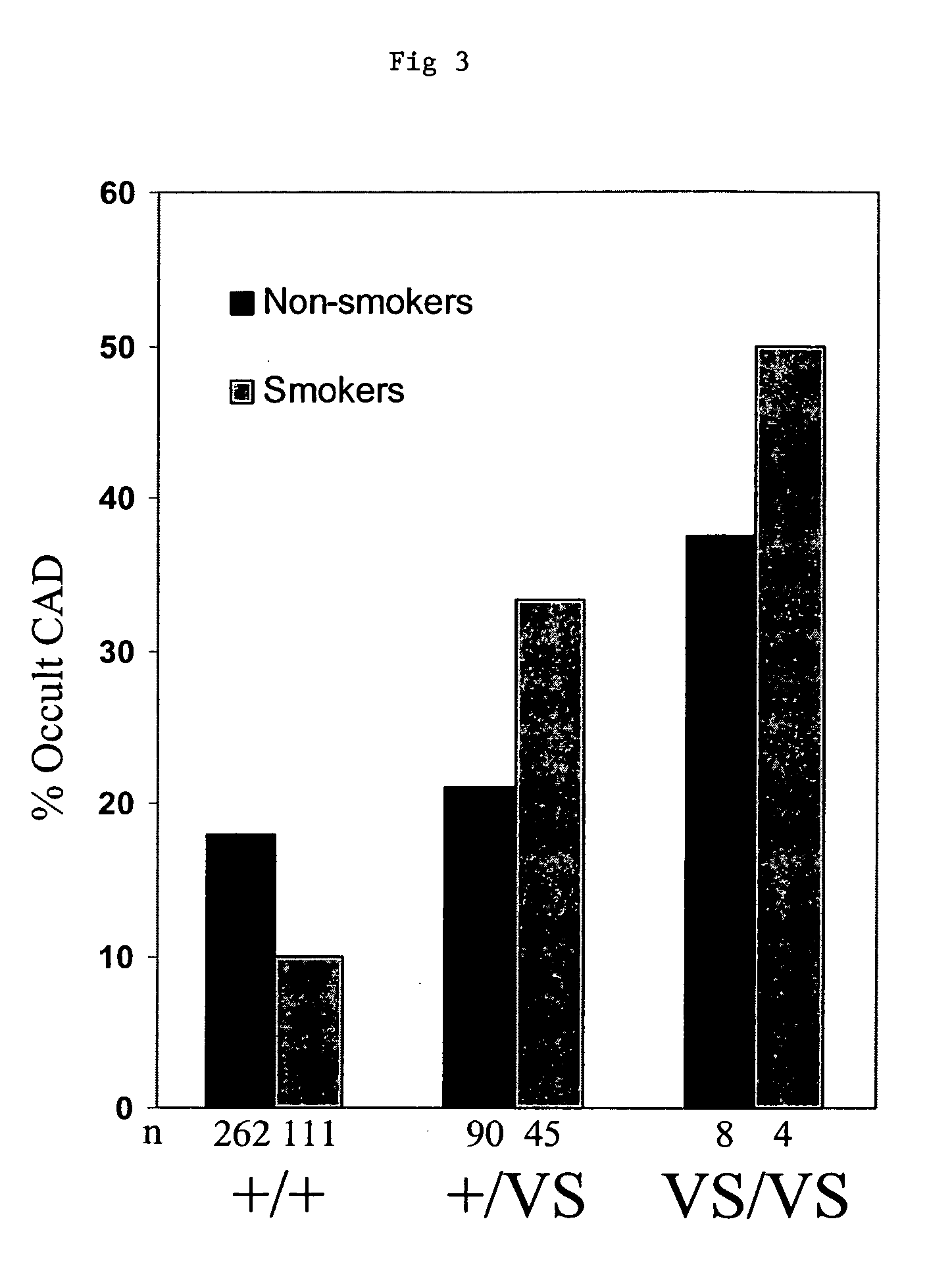
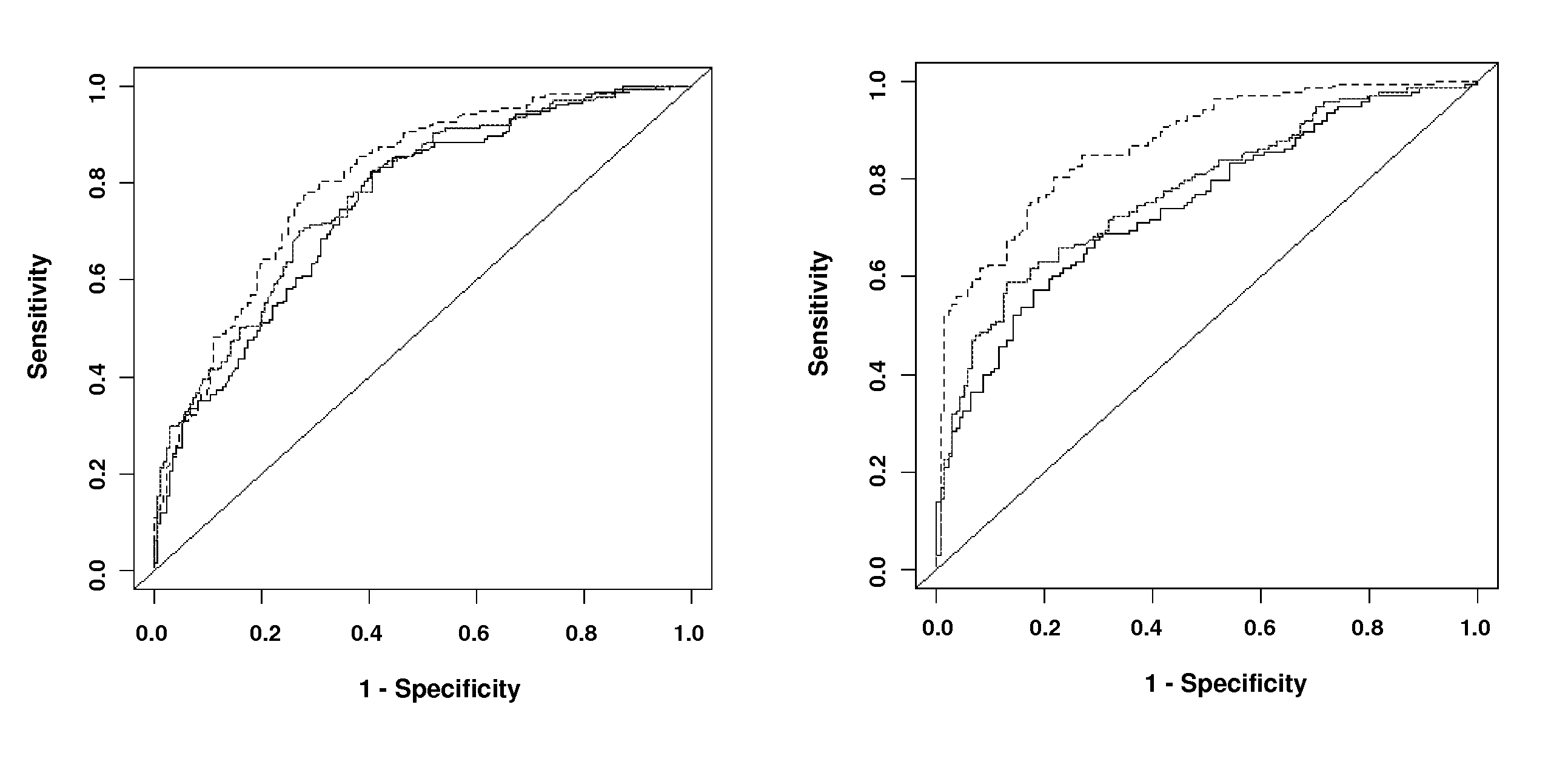
Methods and assays are disclosed for predicting a patient's predisposition for developing coronary artery disease or related vascular disorders. The methods comprise obtaining a biological sample from a patient and determining the presence or absence of the KL-VS allele which is linked with coronary artery disease. Detection of the allele is indicative of a predisposition or propensity to develop coronary artery disease. Kits for the detection of coronary artery disease are additionally provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Predicting coronary artery disease and risk of cardiovascular events
InactiveUS20110318726A1Material analysis by observing effect on chemical indicatorHealth-index calculationCoronary artery diseaseMetabolite
Methods of assessing the risk of cardiovascular disease in a subject by detecting the level of at least one metabolite in a sample from the subject are disclosed herein. The level of the metabolite is indicative of the risk of cardiovascular disease in the subject. The metabolites may be acylcarnitines, amino acids, ketones, free fatty acids or hydroxybutyrate. The cardiovascular disease may be risk of a cardiovascular event, presence of coronary artery disease or risk of development of coronary artery disease.
Owner:DUKE UNIV
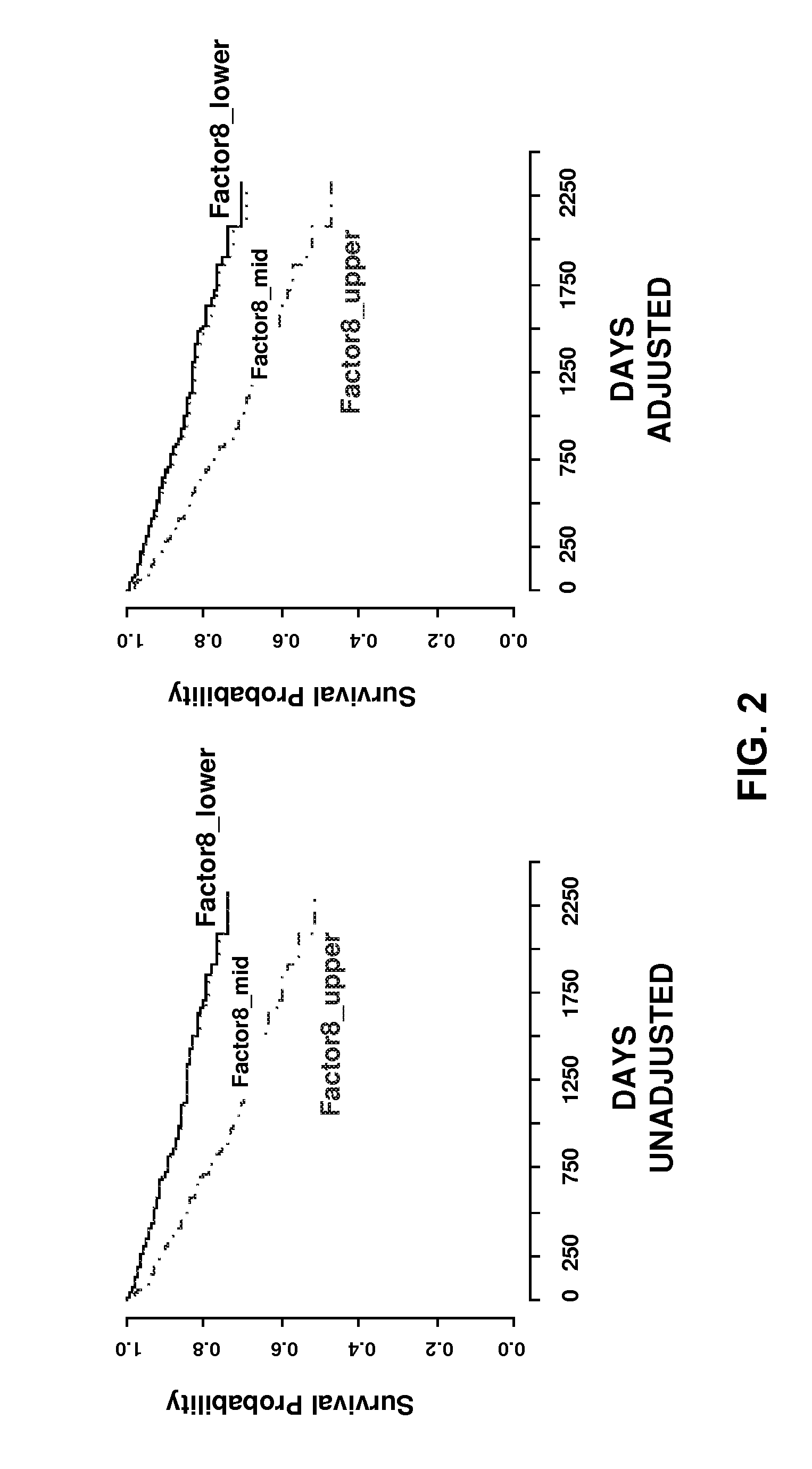
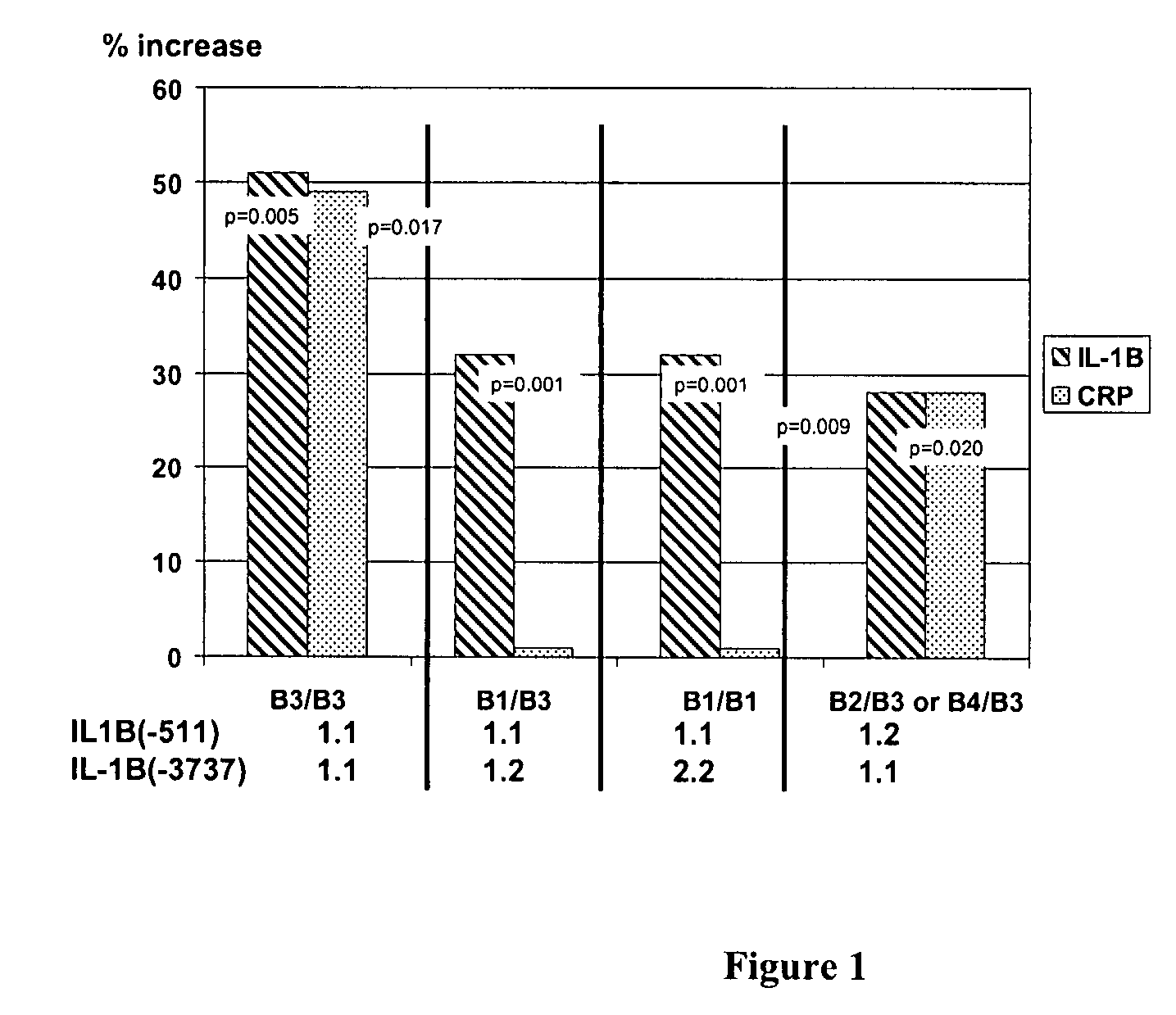
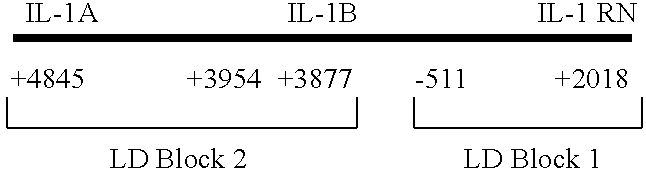
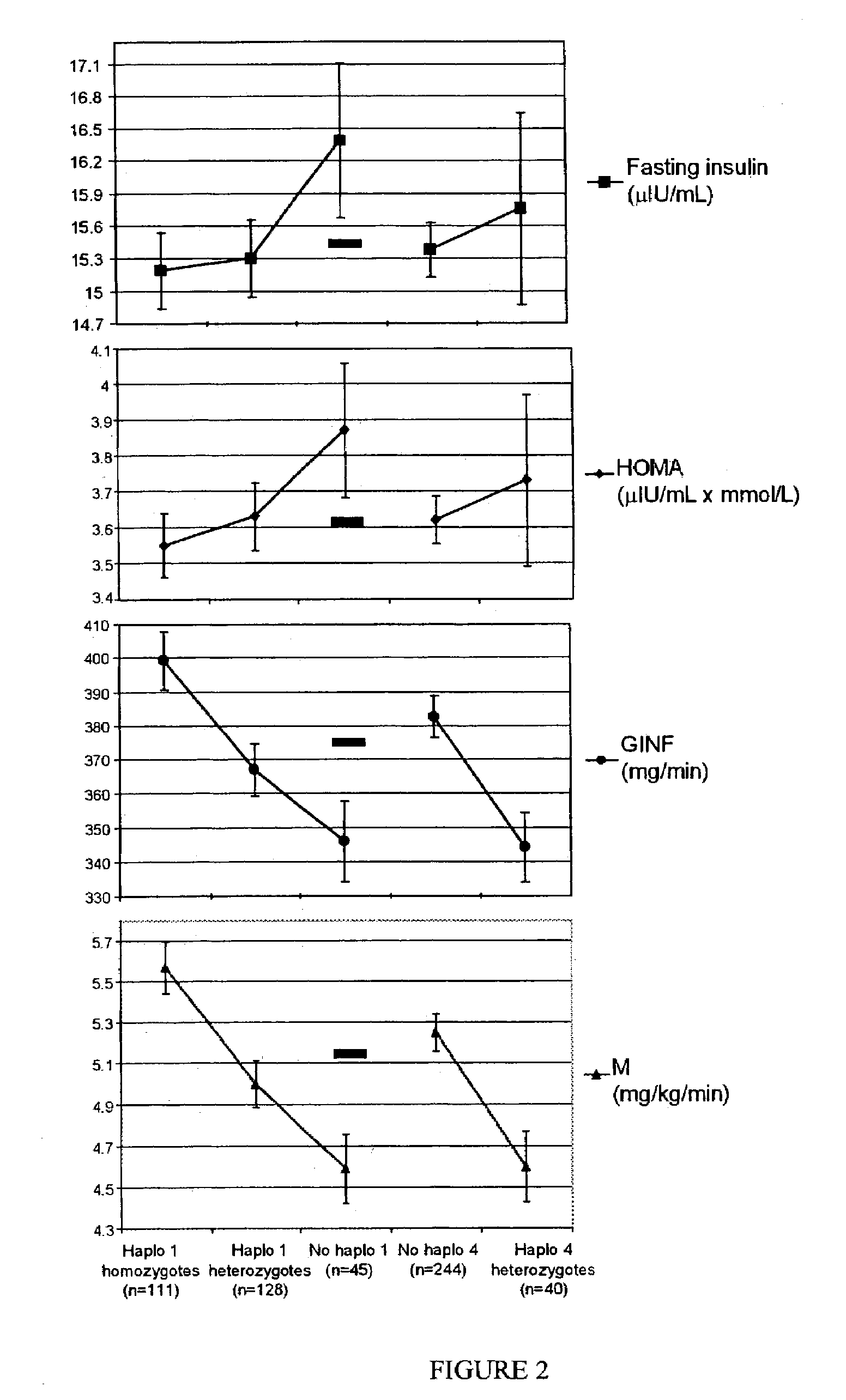
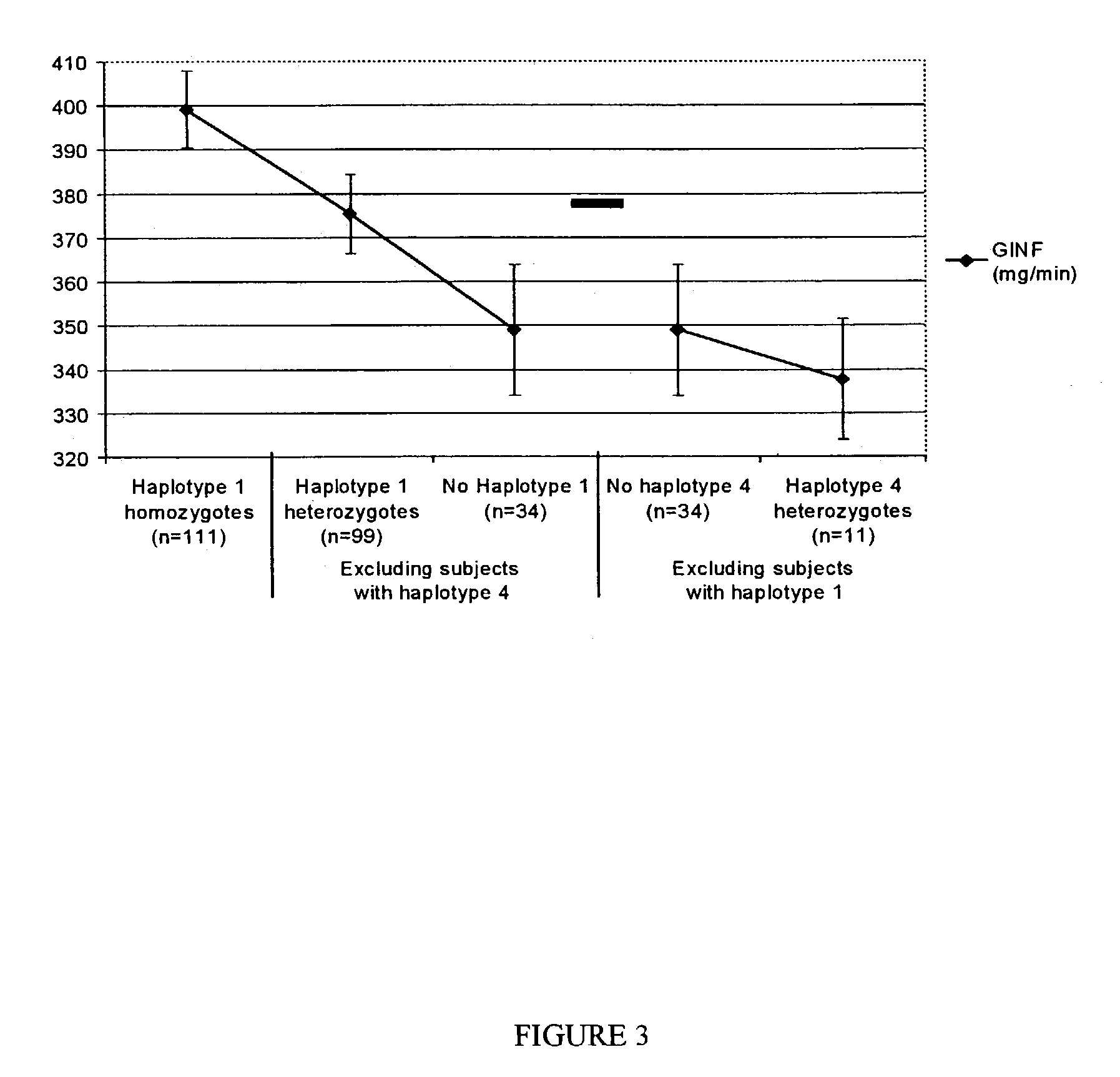
IL-1 gene cluster, insulin resistance and coronary artery disease associated polymorphisms and haplotypes and methods of using same
ActiveUS20090098141A1Improve expression levelSugar derivativesMicrobiological testing/measurementDiseaseCoronary artery disease
The invention provides methods and compositions relating to identification and use of genetic information from the IL-1 gene cluster—including the structure and organization of novel IL-1-like genes found within the IL-1 locus as well as polymorphisms and associated haplotypes within these genes. The invention thereby expands the repertoire of useful genetic information available from the IL-1 locus—which contains the previously-identified IL-1α, IL-1β and IL-1RN genes, for predicting IL-1 associated phenotypes (e.g. increased or decreased risks of insulin resistance associated pathologies) and for treating IL-1 haplotype associated insulin resistance associated pathologies.
Owner:SAPPHIROS LAB LLC
Microporous zirconium silicate for the treatment of hyperkalemia
InactiveUS20160271174A1Reducing scrum potassium levelReduce riskPowder deliveryHeavy metal active ingredientsSide effectTherapeutic treatment
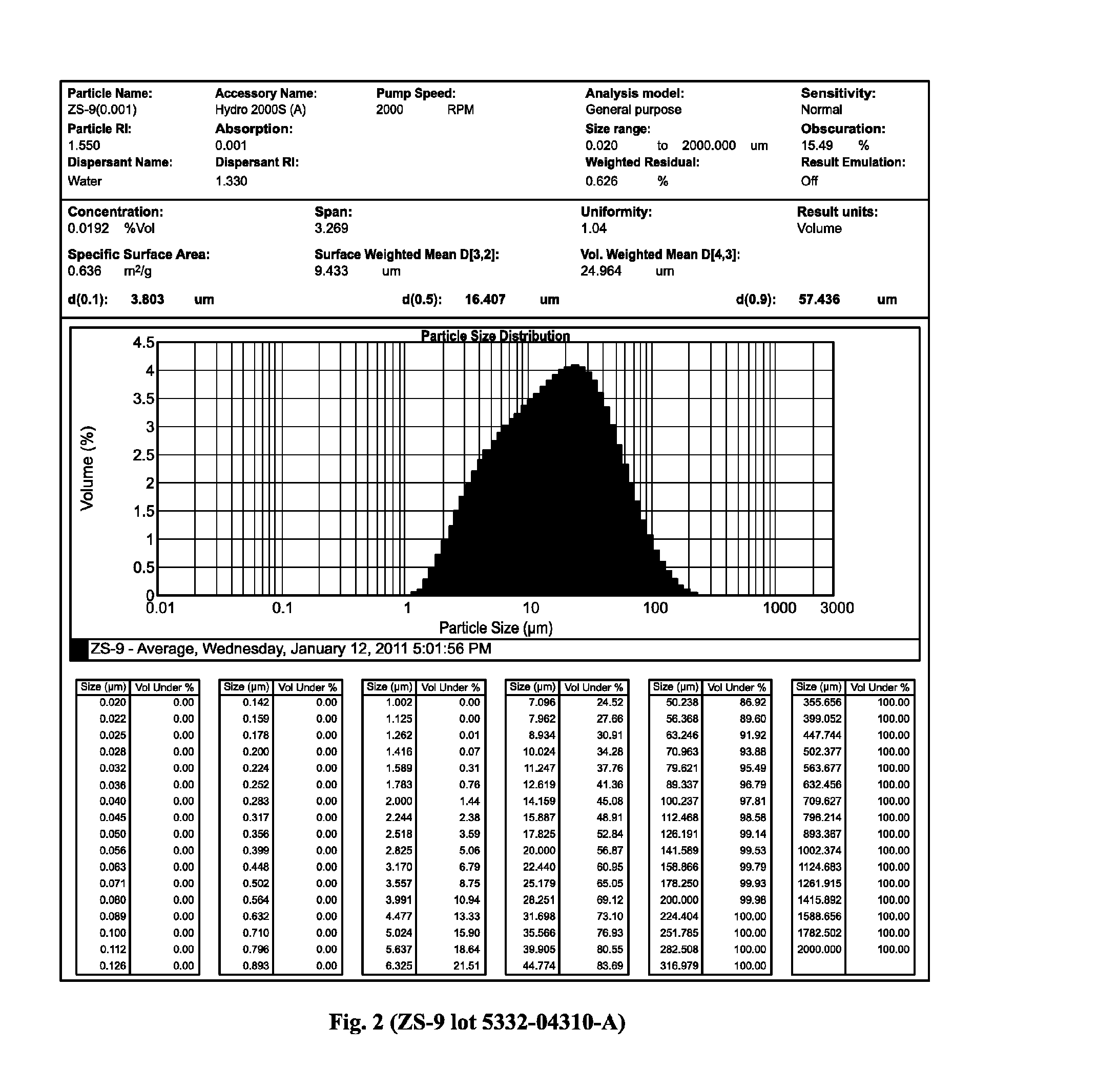
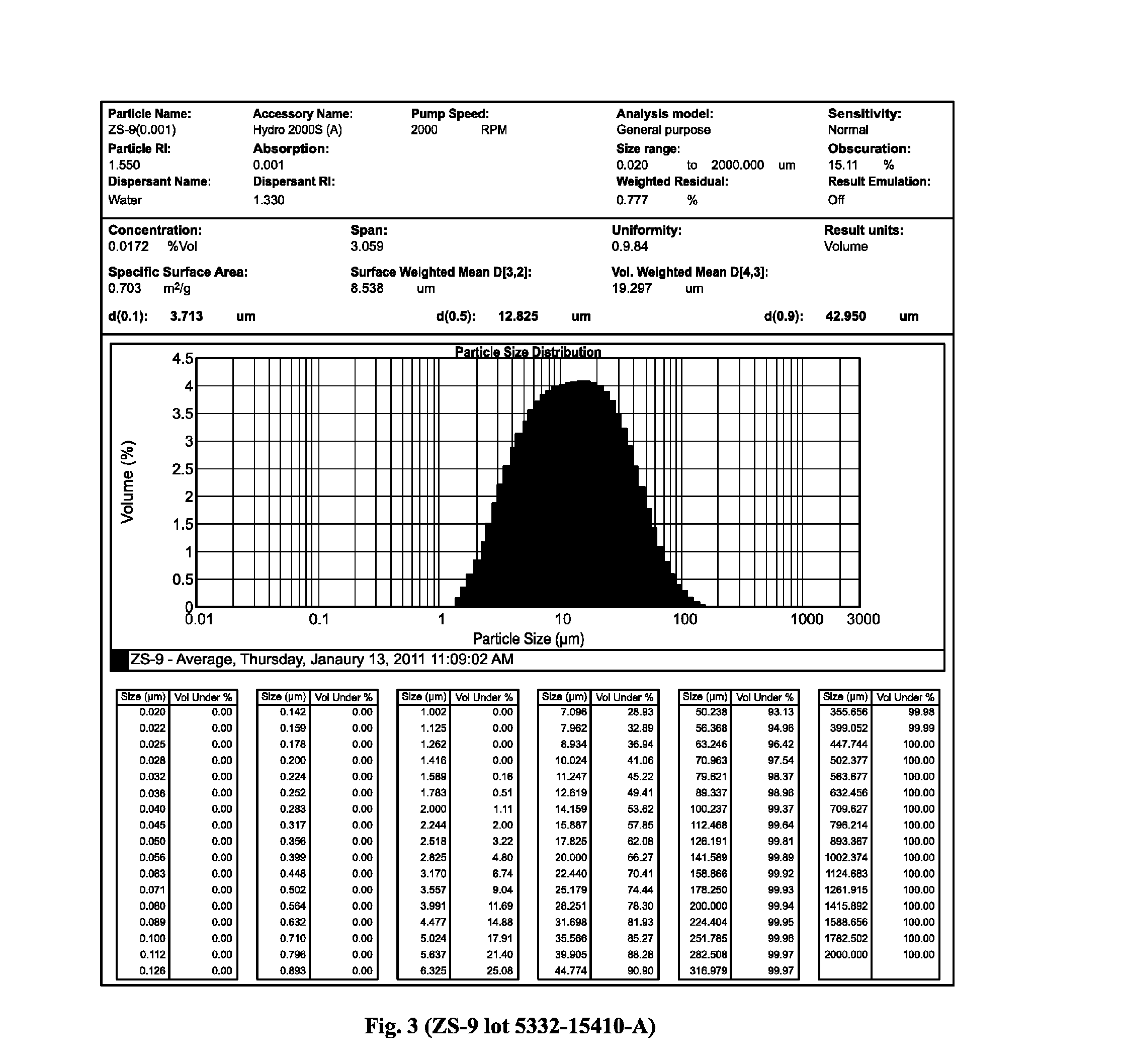
The present invention relates to novel microporous zirconium silicate compositions that are formulated to remove toxins, e.g. potassium ions, from the gastrointestinal tract at an elevated rate without causing undesirable side effects. The preferred formulations are designed avoid increase in pH of urine in patients and / or avoid potential entry of particles into the bloodstream of the patient. Also disclosed is a method for preparing high purity crystals of ZS-9 exhibiting an enhanced level of potassium exchange capacity. These compositions are particularly useful in the therapeutic treatment of hyperkalemia. These compositions are also useful in the treatment of chronic kidney disease, coronary vascular disease, diabetes mellitus, and transplant rejection.
Owner:ZS PHARMA
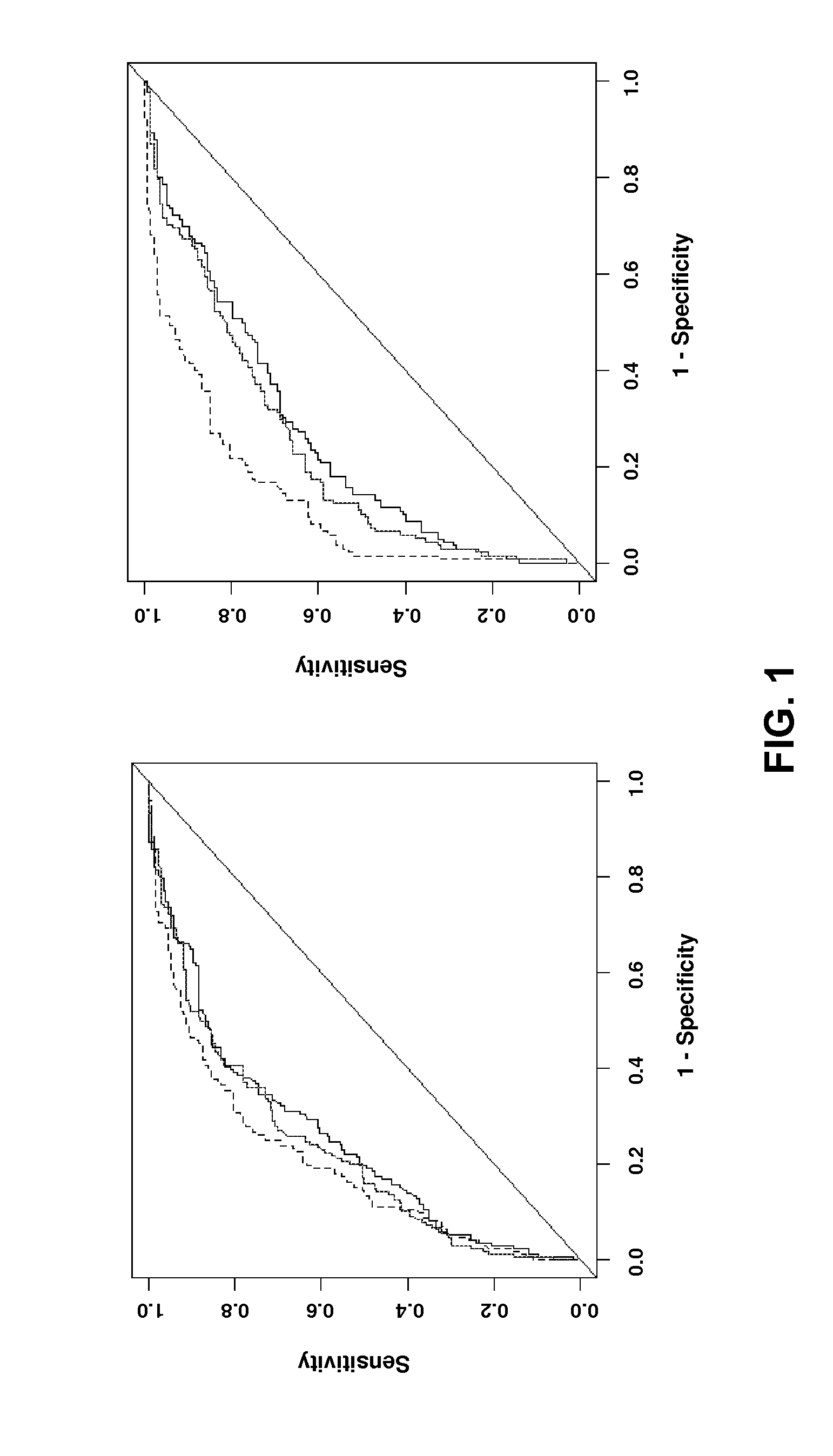
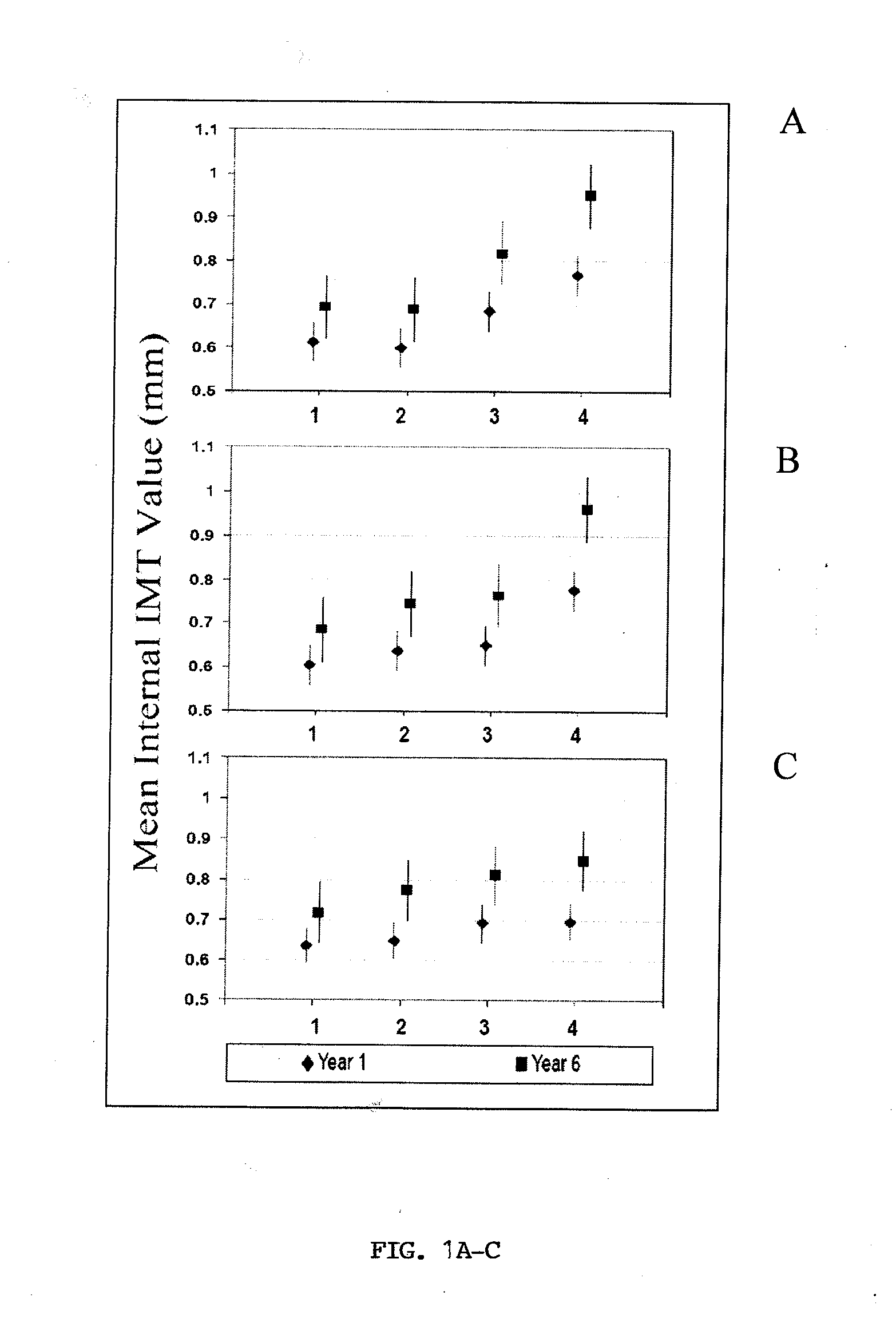
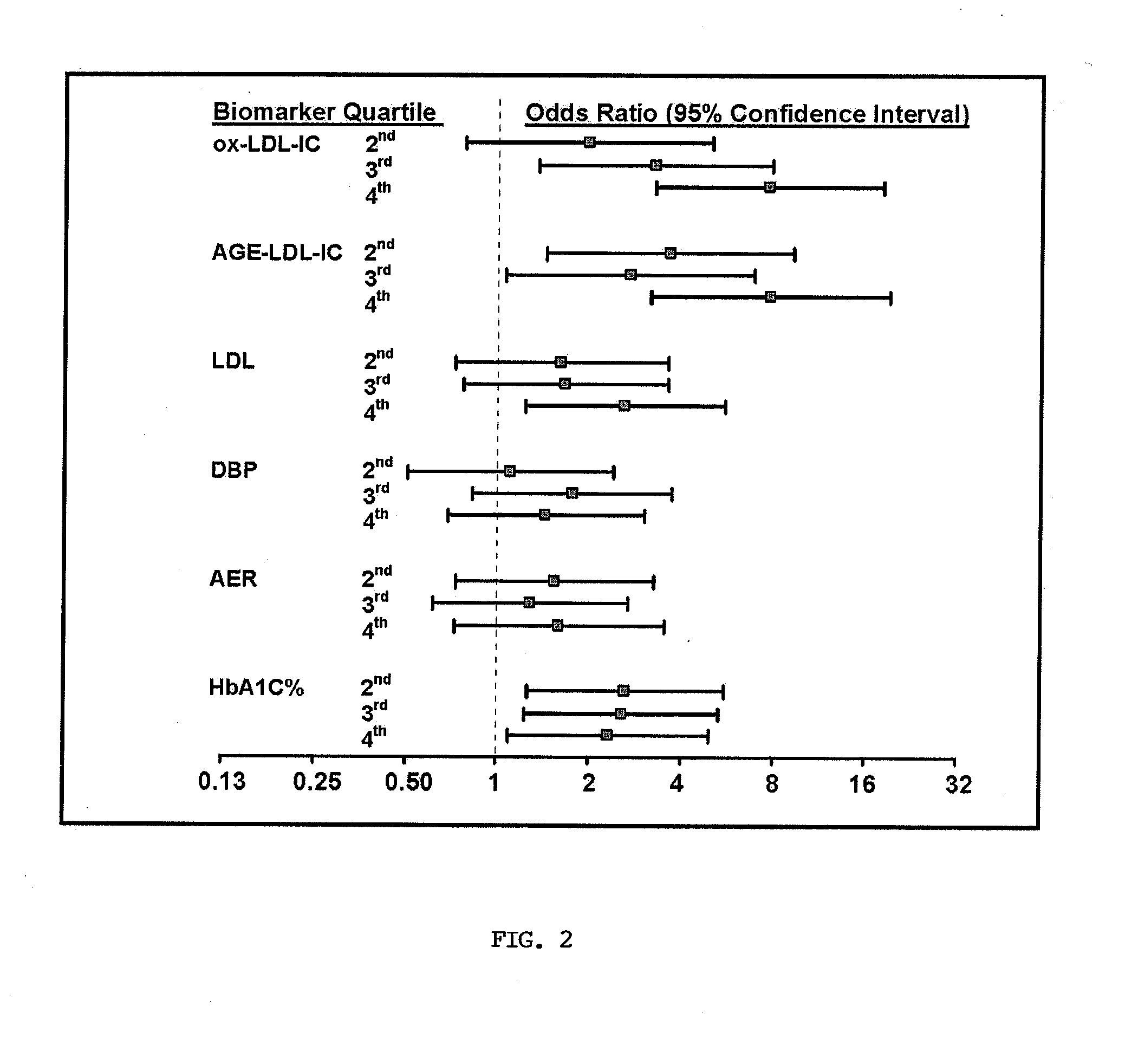
Methods For Assessing Modified LDL Immune Complexes in Subjects Having or at Risk of Coronary Artery Disease
The present invention relates to the analysis of modified LDL in the context of immune complexes. In particular, ox-LDL and AGE-LDL are shown to predict the development of coronary artery disease and other micro- and macrovascular disorders, particularly in the context of diabetes.
Owner:MUSC FOUND FOR RES DEV +1
Biomarkers for coronary artery disease
ActiveCN107075453AGuaranteed complianceGuaranteed accuracyBacteriaMicrobiological testing/measurementCoronary heart diseaseHeart disease
Biomarkers and methods for predicting the risk of a disease related to microbes are provided, in particular coronary artery disease (CAD) or related heart diseases.
Owner:BGI SHENZHEN CO LTD +1
Kit for estimating coronary artery diseases
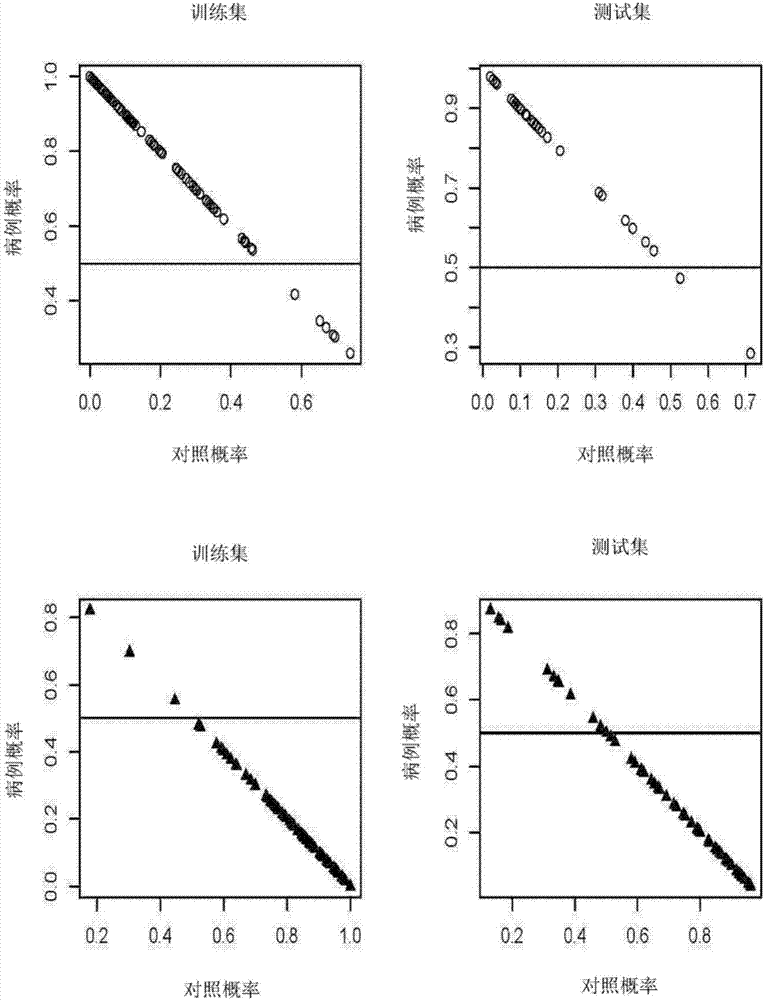
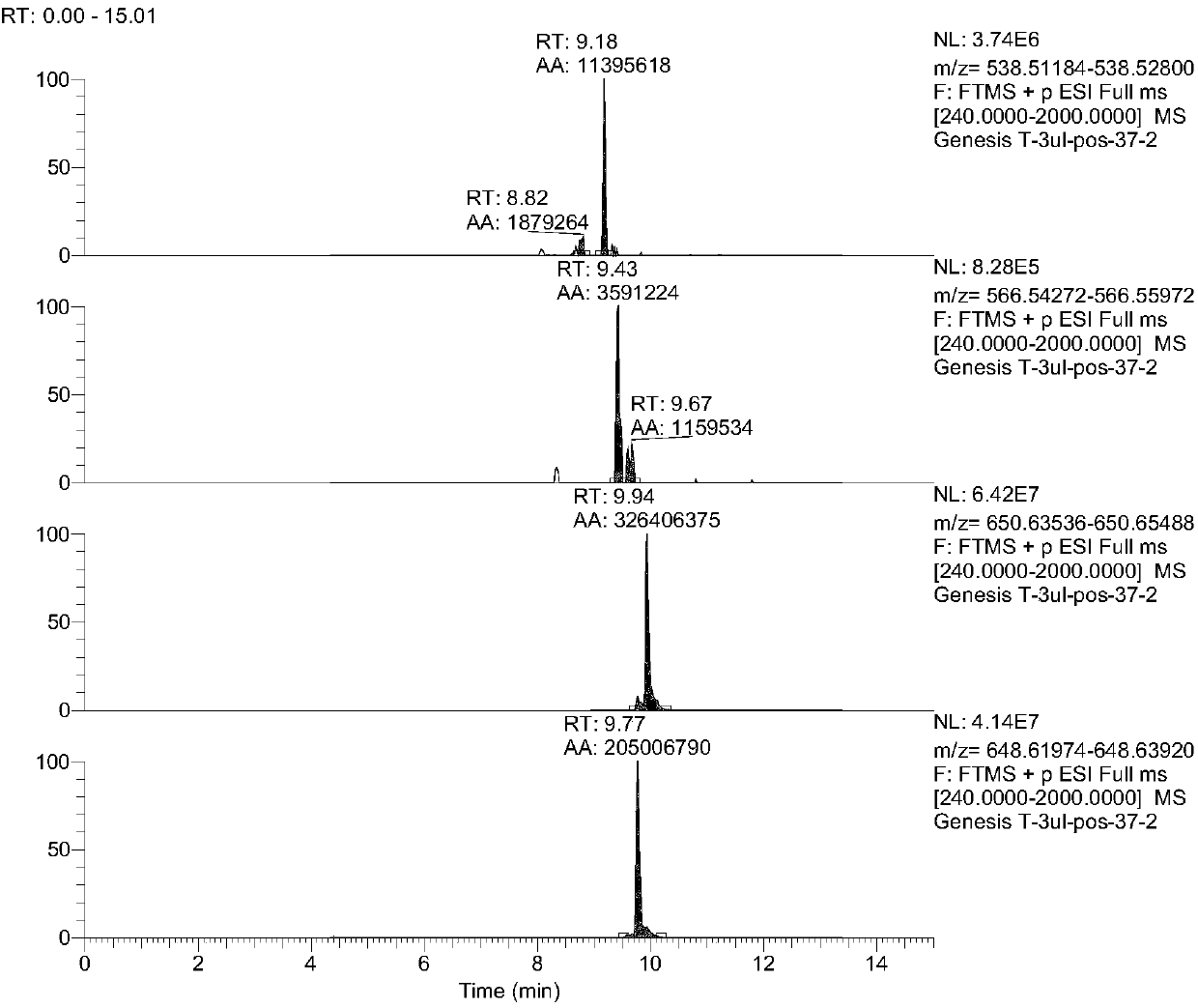
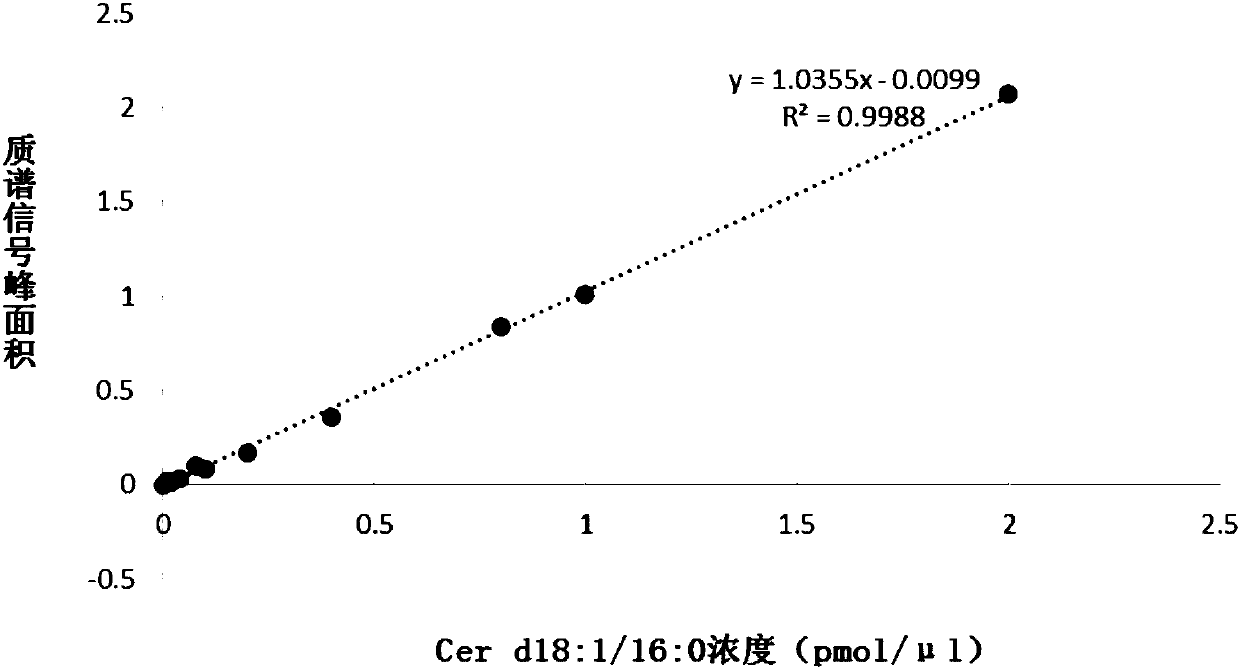
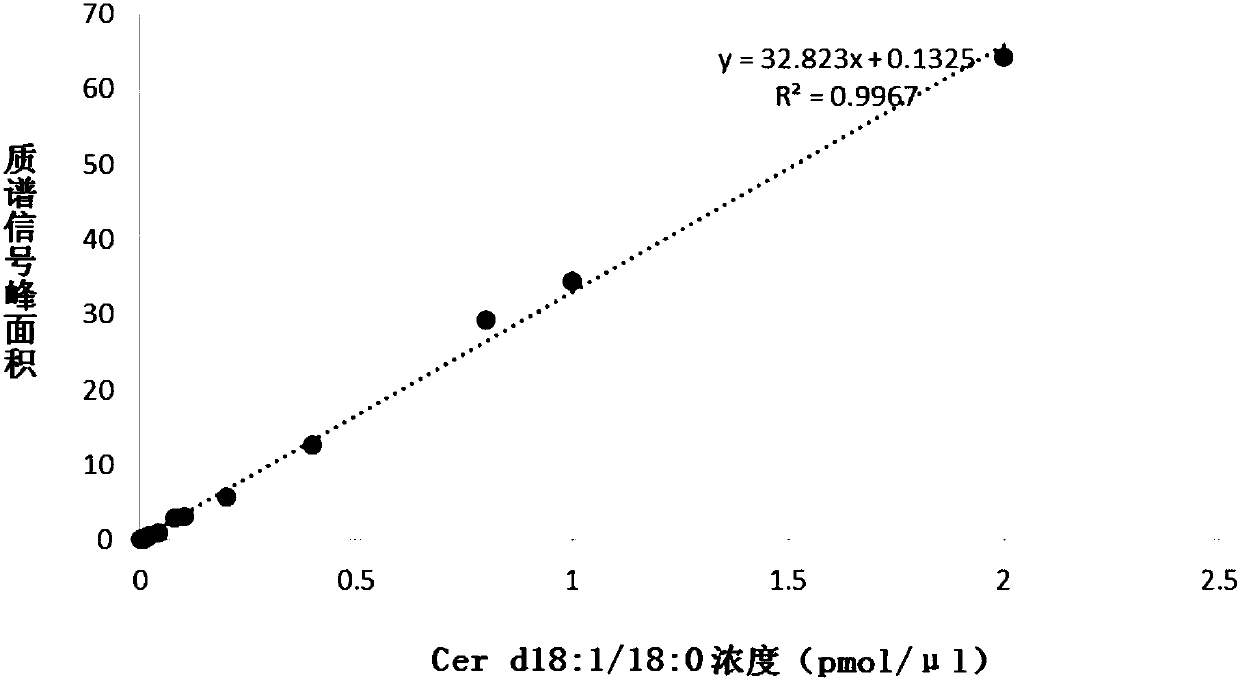
PendingCN108037275AEffective diagnosisAccurate diagnosisComponent separationBiological testingCoronary arteriesTherapeutic evaluation
The invention provides a kit for estimating coronary artery diseases. The kit comprises a detection reagent for detecting concentrations of Cer(d18:1 / 16:0), Cer(d18:1 / 18:0), Cer(d18:1 / 24:0) and Cer(d18:1 / 24) in a sample of a study subject and a specification. The kit can be efficiently and accurately used for the diagnosis, risk stratification, illness monitoring and curative effect evaluation ofthe coronary artery diseases, is good in sensitivity and specificity and can meet the clinical diagnosis and treatment requirement of the coronary artery diseases.
Owner:江苏豪思生物科技有限公司
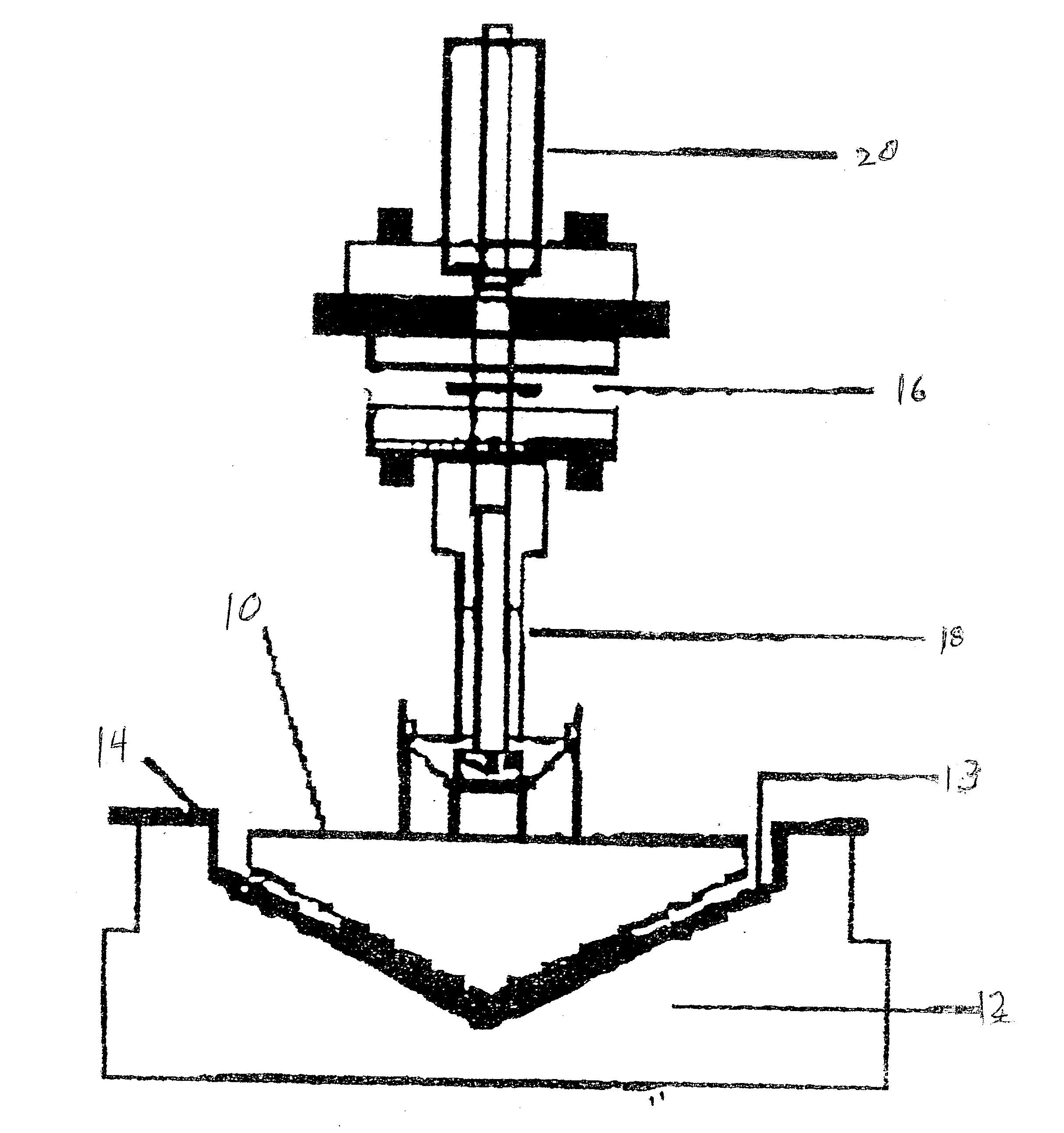
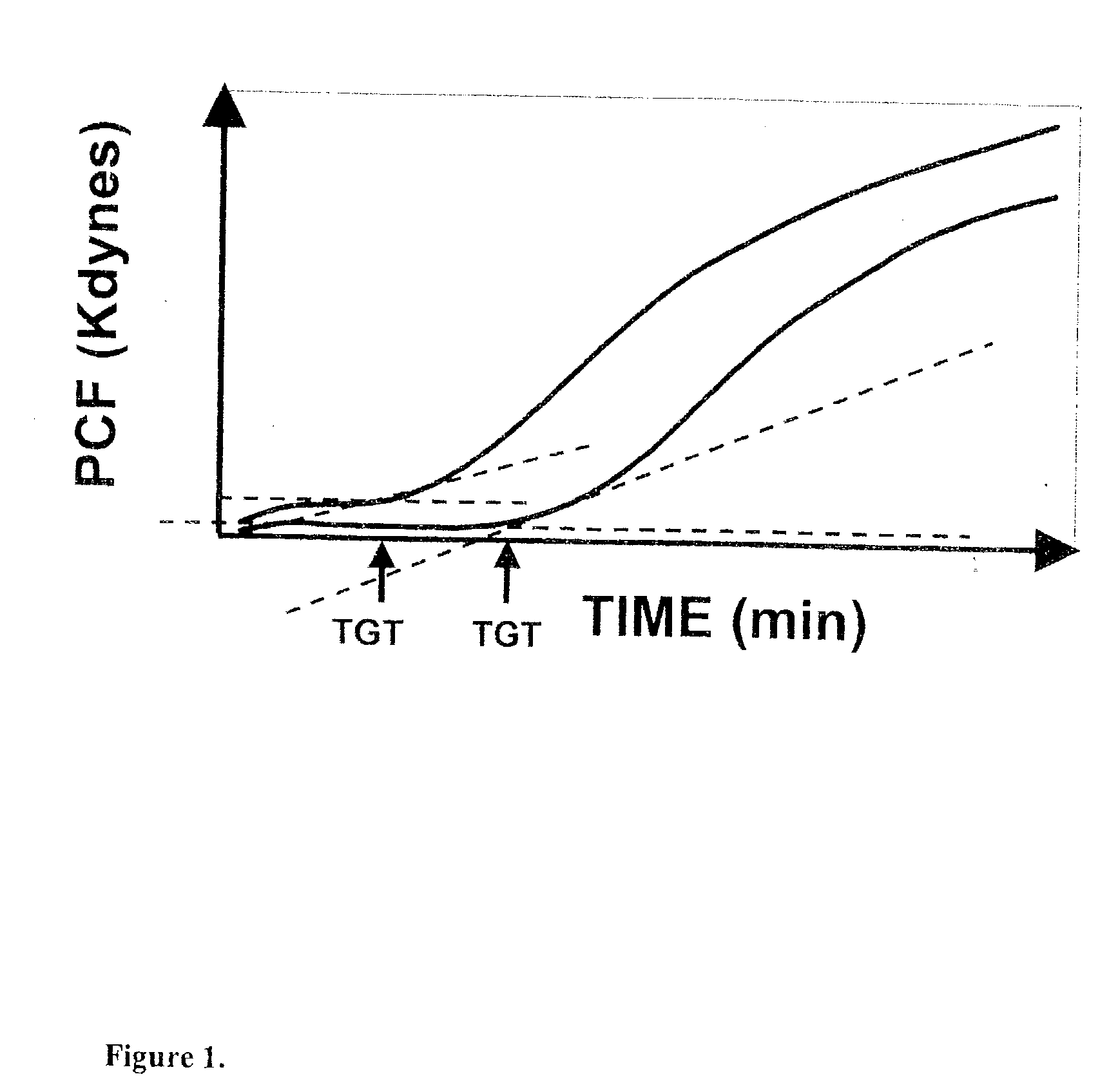
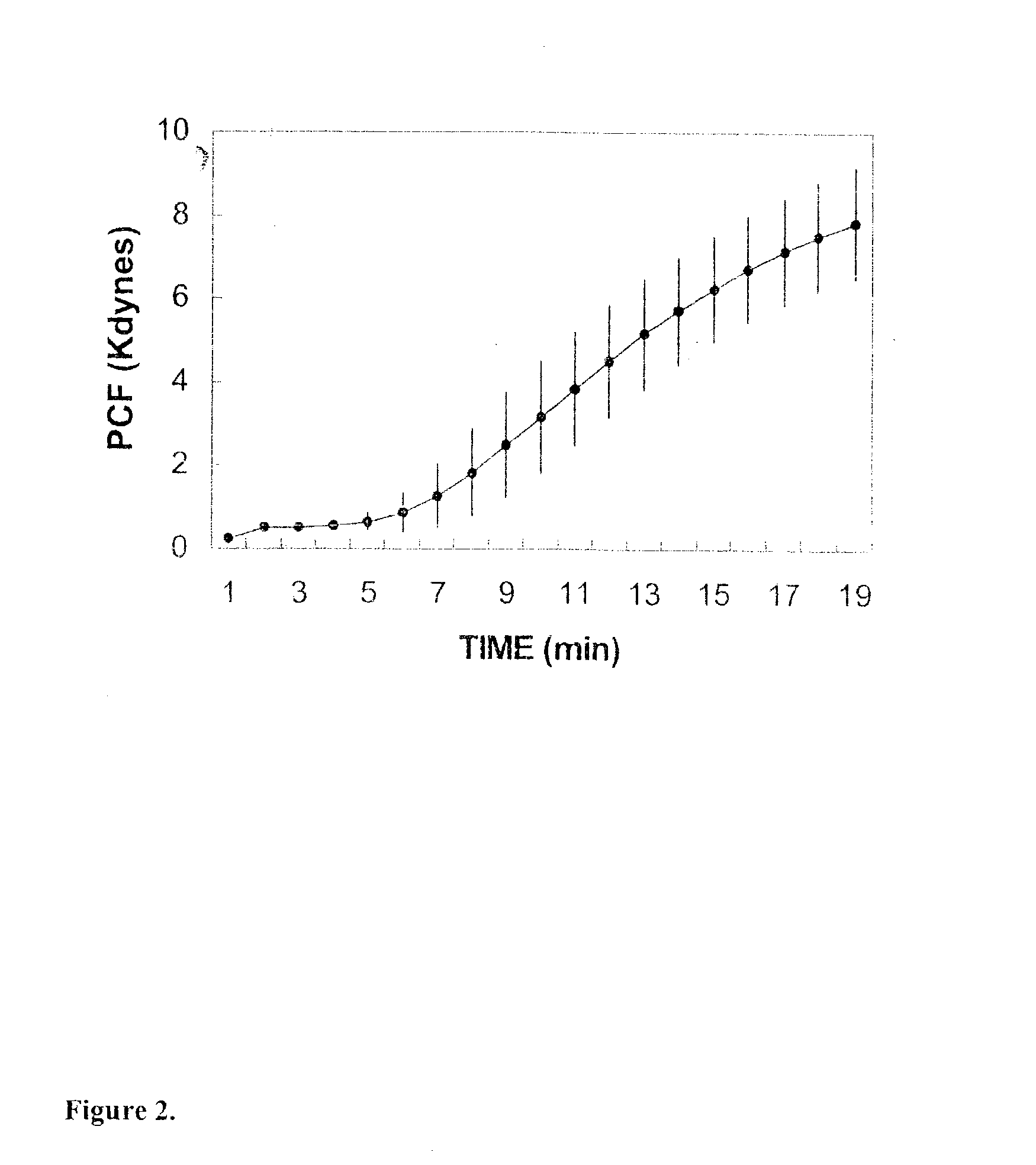
Onset of force development as a marker of thrombin generation
InactiveUS20030199428A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseCoronary artery disease
Platelet contractile force (PCF) is used as a surrogate marker of thrombin generation. PCF generation occurs concomitant with the burst of prothrombin fragment F 1+2 release. The time between assay start and PCF onset is identified as the thrombin generation time (TGT), and is used in assessing risk of bleeding, in diagnosing various disorders, and in monitoring the effects of pharmaceutical and other treatments. TGT is prolonged in clotting factor deficiencies and in the presence of direct and indirect thrombin inhibitors. TGT shortens to normal with clotting factor replacement and shortens with administration of rVIIa. TGT is short in thrombophilic states such as coronary artery disease, diabetes and thromboangiitis obliterans and prolongs toward normal with oral and intravenous anticoagulants.
Owner:HEMODYNE
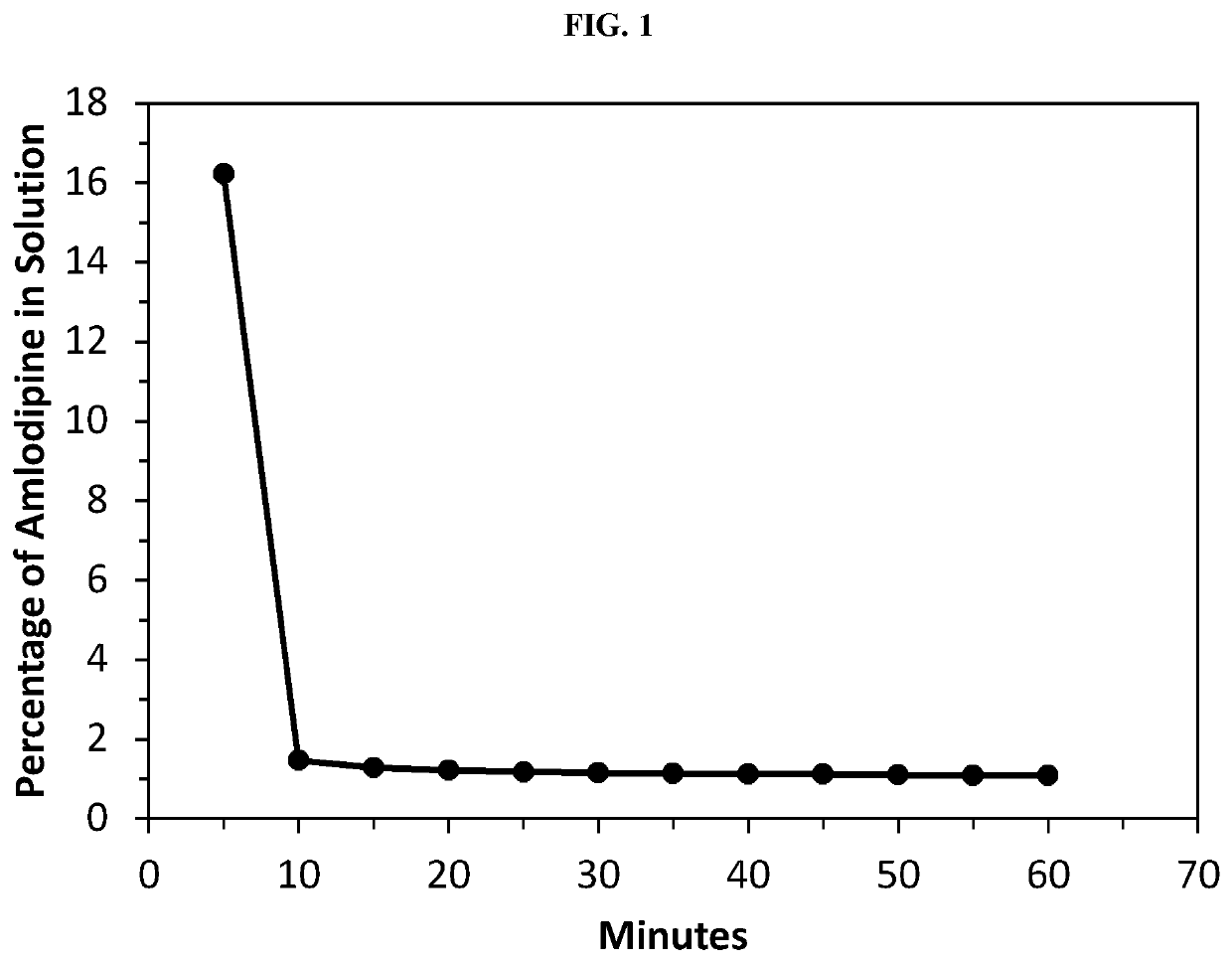
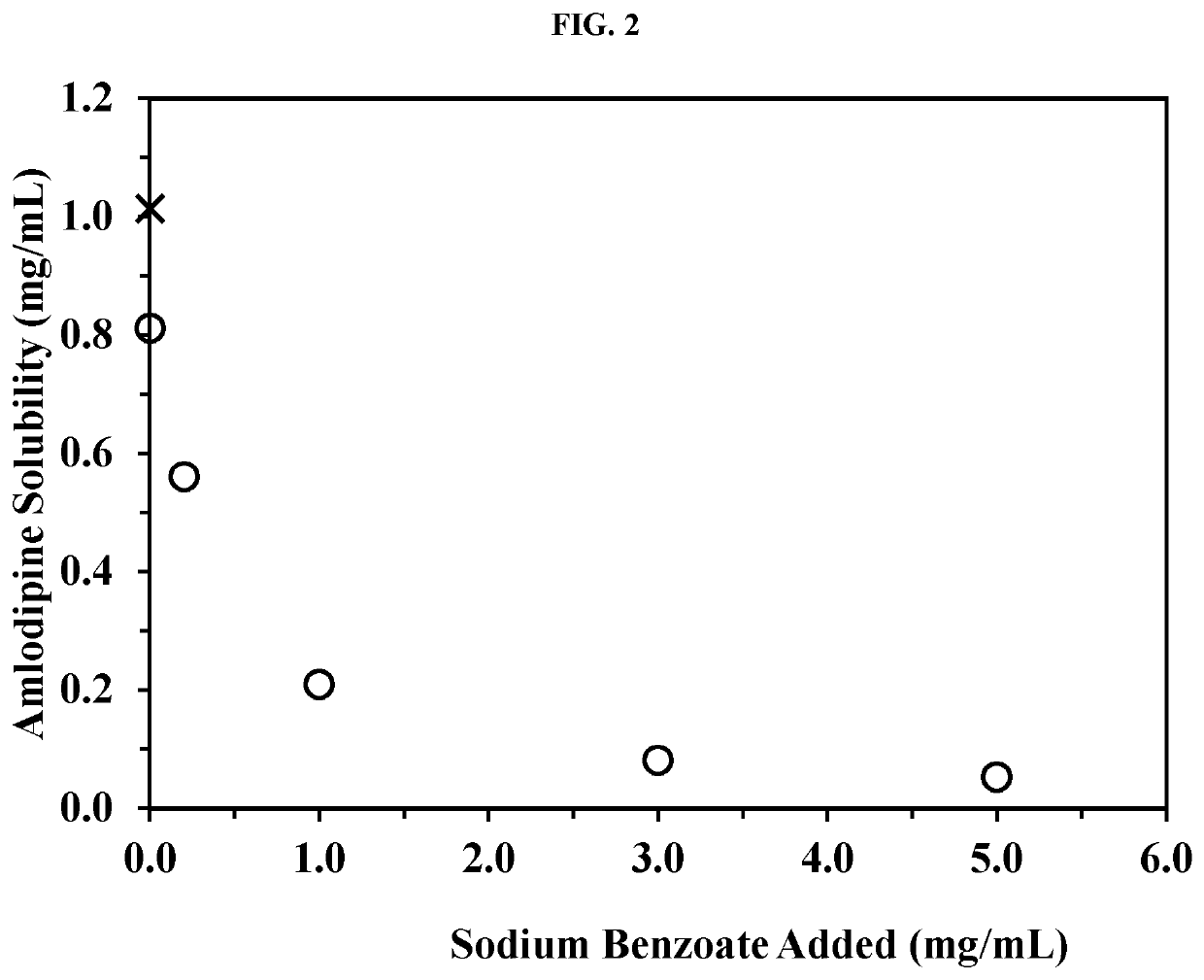
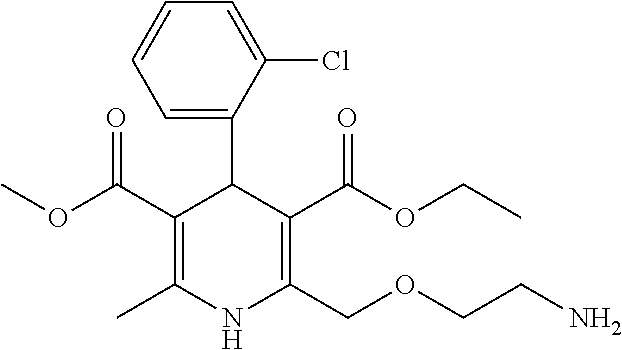
Amlodipine formulations
Owner:AZURITY PHARMA INC
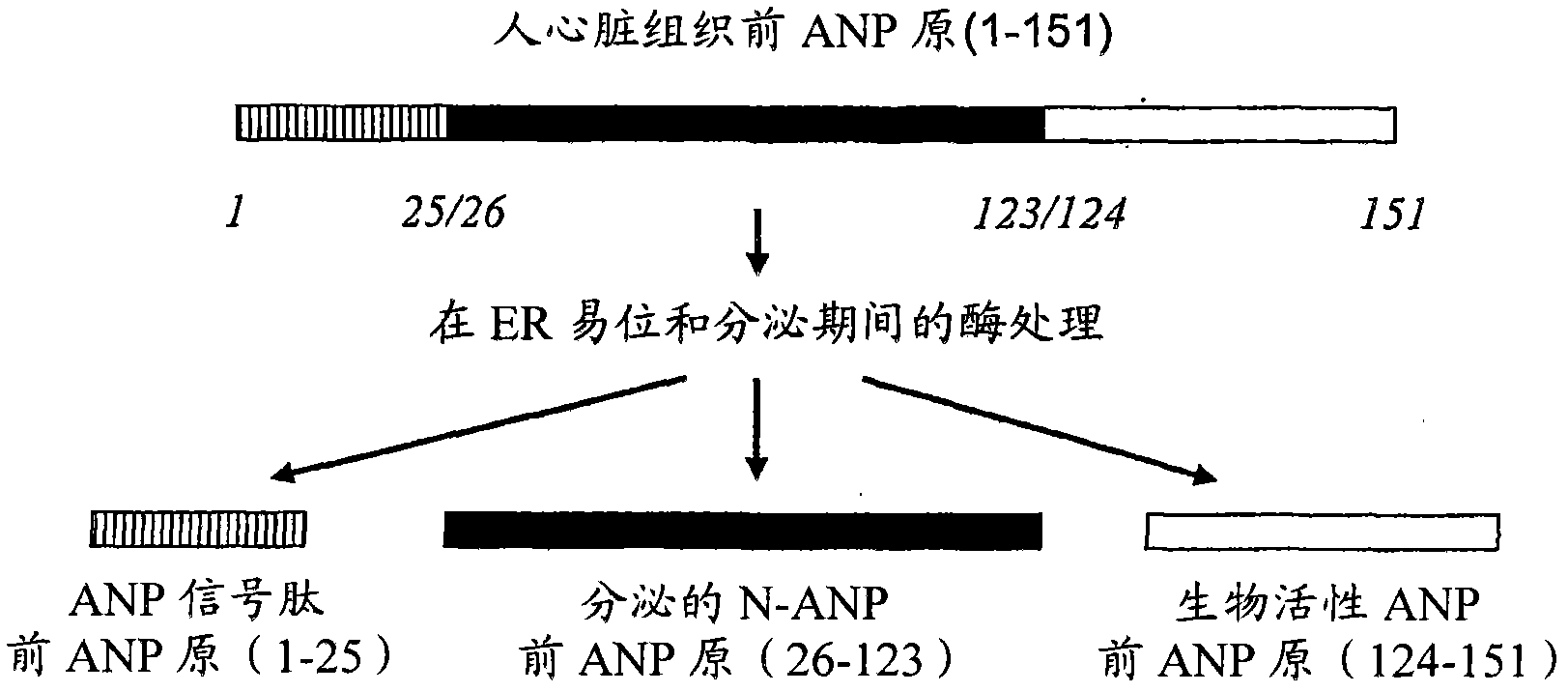
Biomarkers for acute coronary disorder
The invention provides methods for predicting, diagnosing or monitoring acute cardiac disorders, cardiac transplant rejection, or distinguishing acute cardiac disorders from pulmonary disorders, by measuring ANP signal peptide levels in a sample taken from a subject shortly after onset of, or presentation with the disorder of transplant rejection. Also provided are antibodies useful in the methods of the invention.
Owner:OTAGO INNOVATION
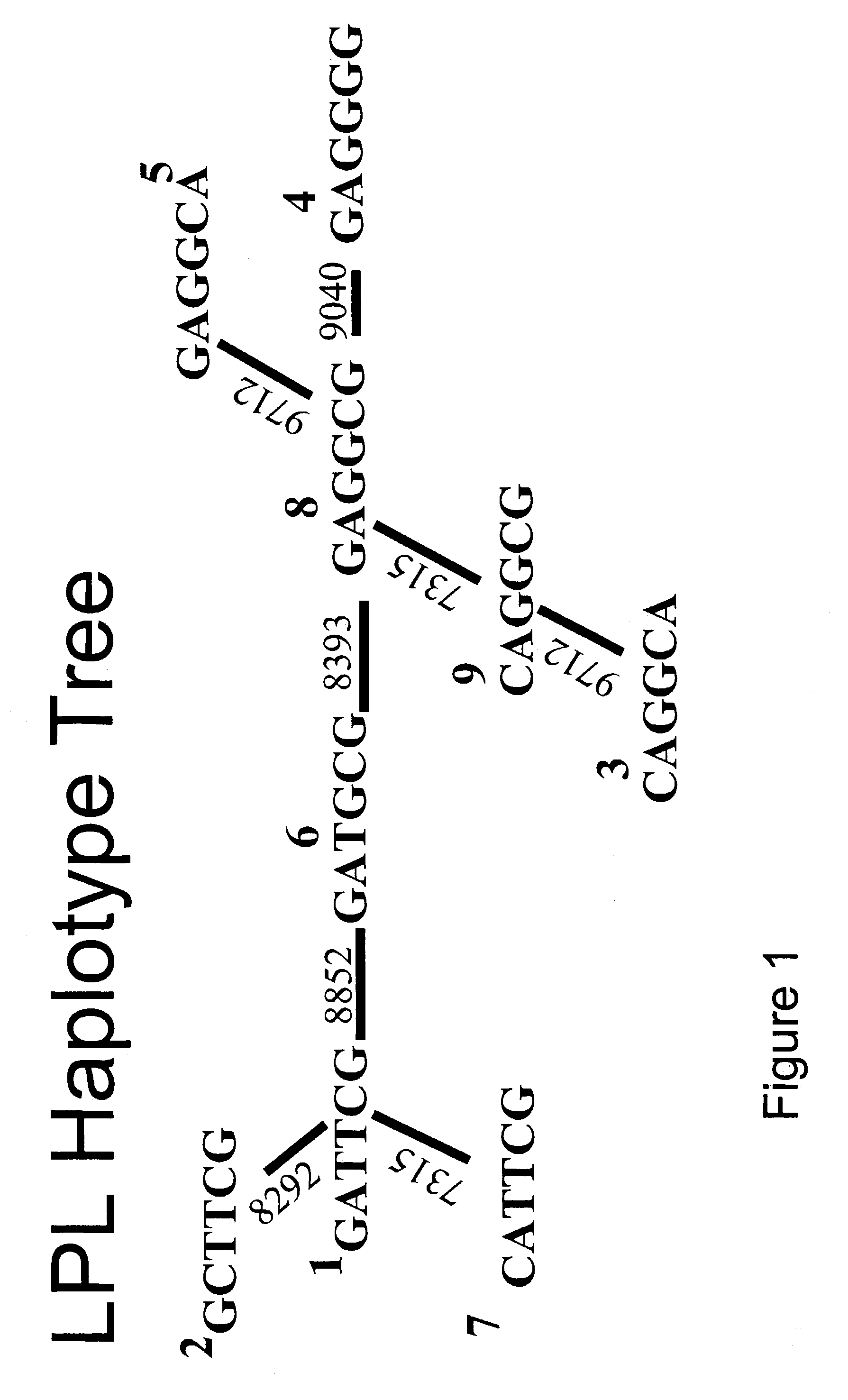
Method of haplotype-based genetic analysis for determining risk for developing insulin resistance and coronary artery disease
InactiveUS7141373B2Sugar derivativesMicrobiological testing/measurementGenetics predispositionGenetics
Disclosed is a method for determining haplotypes useful for large-scale genetic analysis, within a genomic reference sequence of interest, for a human subpopulation. The method can applied to statistically evaluating the genotypes of subjects for any statistically significant association with a phenotype of interest, such as insulin resistance or coronary artery disease. Thus, also disclosed are a method of detecting a genetic predisposition in a Mexican-American human subject for developing insulin resistance and methods of detecting a lower than normal risk in a Mexican-American human subject for developing insulin resistance or coronary artery disease.
Owner:RGT UNIV OF CALIFORNIA +1
Methods and pharmaceuticals compositions for treating coronary artery disease, ischemia,and vascular disease using angiopoietins
InactiveUS7427594B1Promoting angiogenesis and endothelial survivalImprove bindingPeptide/protein ingredientsAngiogeninVascular diseaseNucleic acid molecule
Pharmaceutical compositions that comprise a pharmaceutically acceptable carrier and either a therapeutically effective amount of an ECM-binding fragment of Ang-1 protein that comprises SEQ ID NO:1 and / or SEQ ID NO:2 or a homologous peptide thereof and pharmaceutical compositions that comprise a pharmaceutically acceptable carrier and a vector comprising a nucleic acid molecule that comprises the nucleotide sequence that encodes an ECM-binding fragment of Ang-1 protein that comprises SEQ ID NO:1 and / or SEQ ID NO:2 or a homologous peptide thereof are disclosed. Methods of using such compositions to treat individuals suspected of having coronary artery disease, vascular disease or a condition involving ischemia and to promote angiogenesis, endothelial survival and maintaining vascular integrity are disclosed. Methods to identify compounds that modulates binding of Ang-1 to ECM are disclosed. Pharmaceutical compositions which comprise a therapeutically effective amount of Ang-2 protein and / or a vector comprising a nucleic acid molecule that comprises the nucleotide coding sequence of Ang-2 and methods of using such compositions to treat individuals suspected of having cancer are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Pro-angiogenic peptides and peptide conjugates
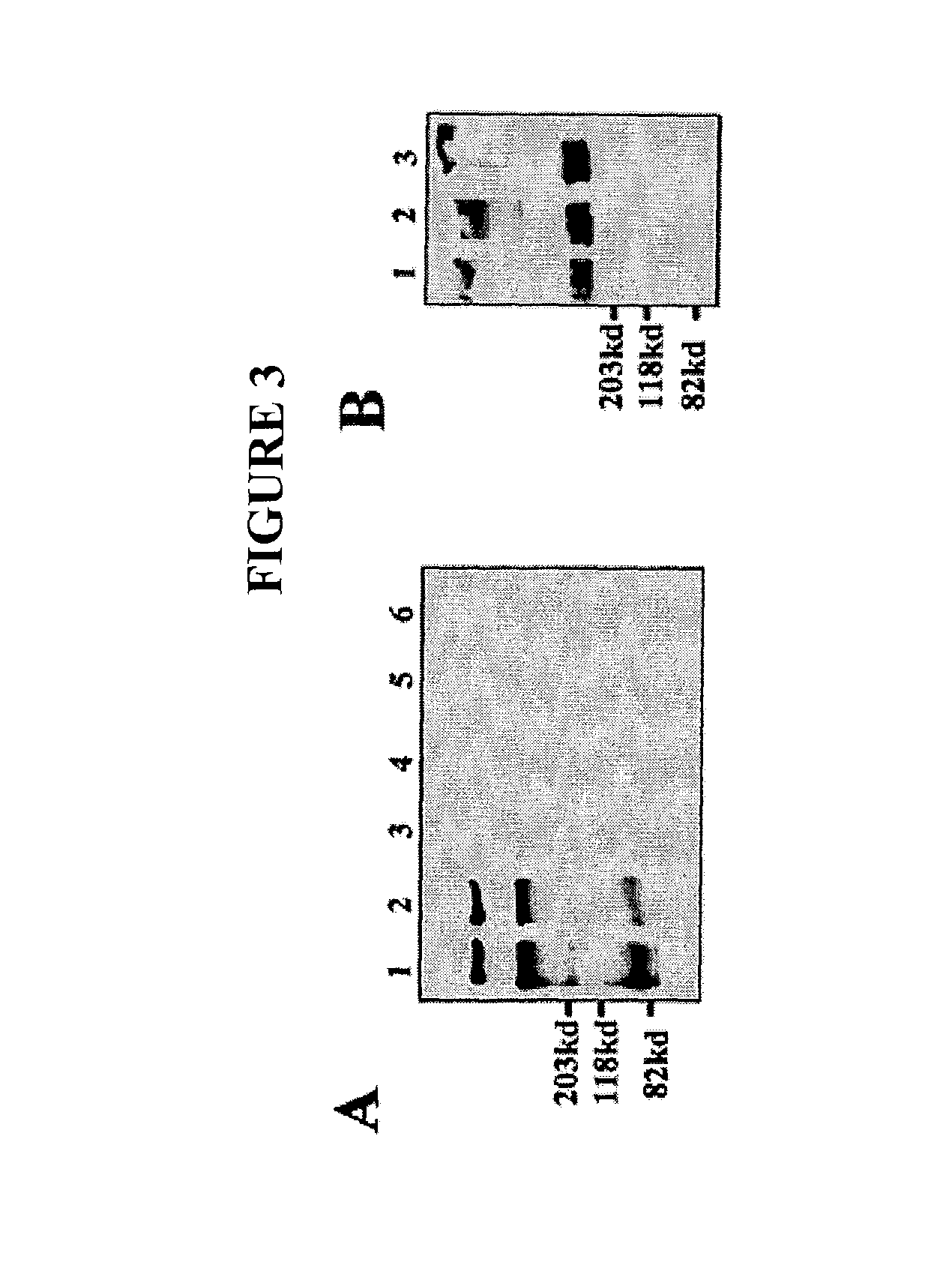
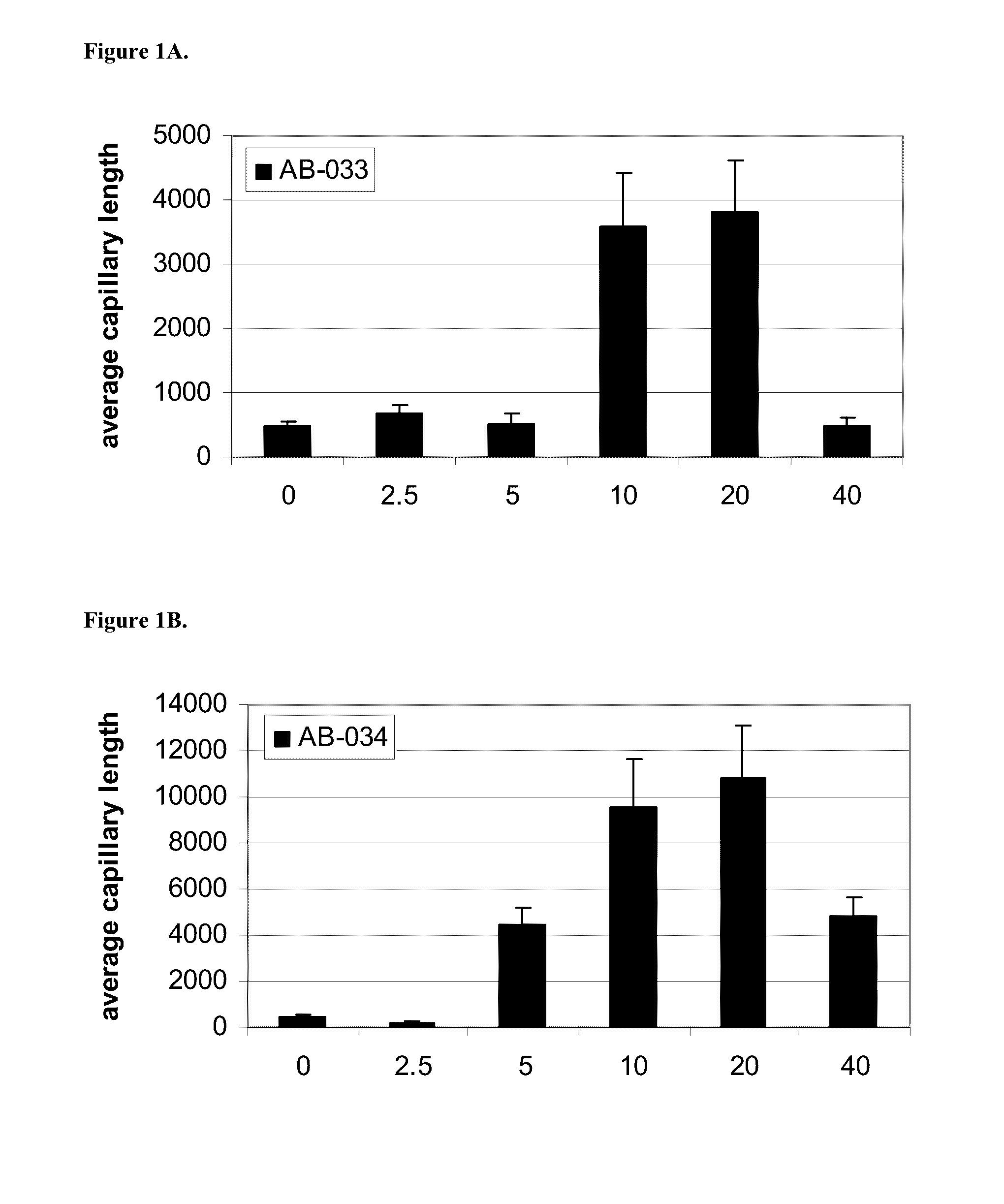
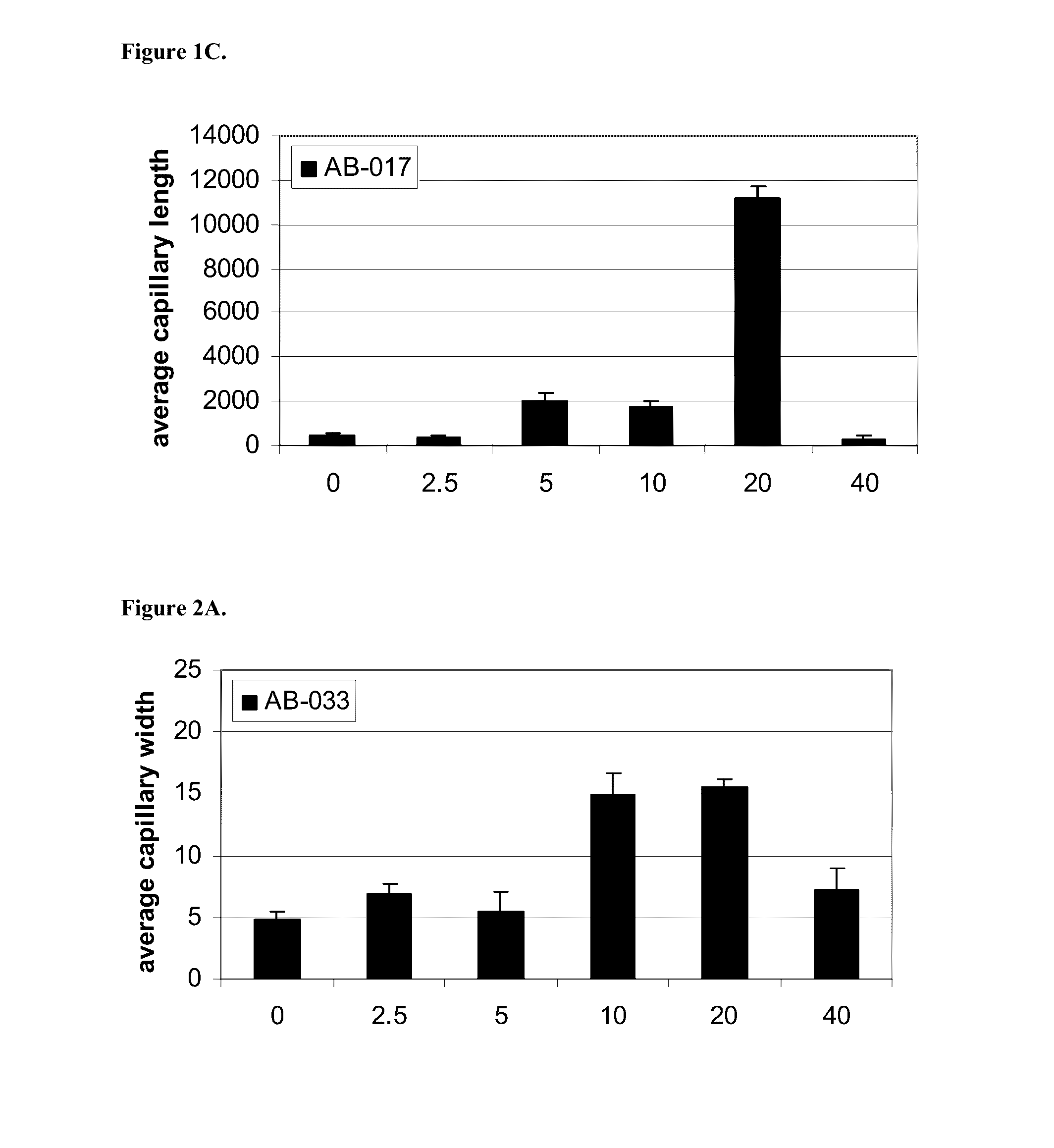
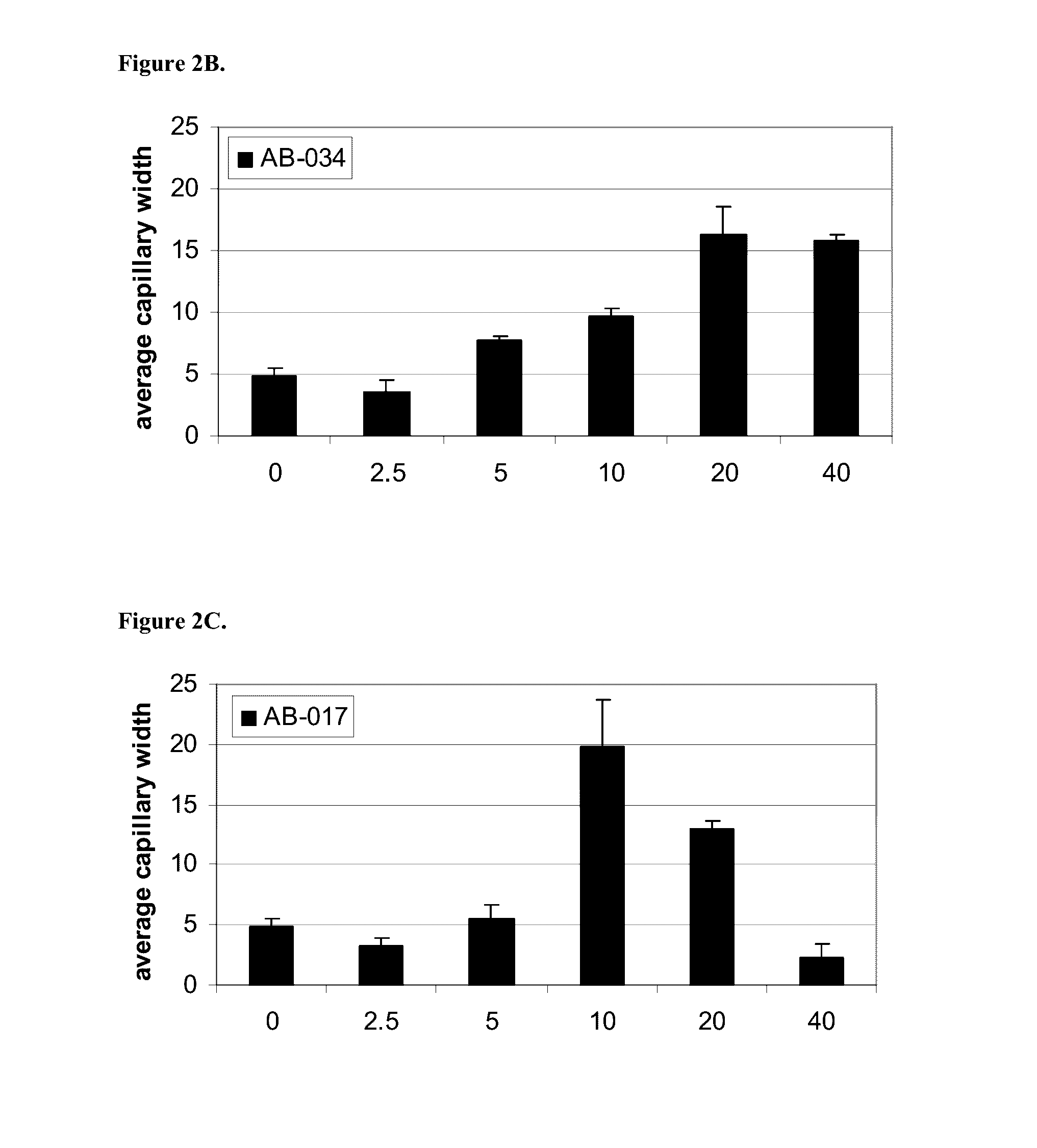
InactiveUS20160333070A1Promote angiogenesisPromote growthSenses disorderDispersion deliveryReceptorBlood vessel
Short bioactive sequences derived from the 2nd loop of the 7-transmembranal receptor of endothelial differentiation gene 3 (EDG3) useful in stimulation of angiogenesis, and peptide conjugates comprising a permeability enhancing moiety, are provided. Also provided are pharmaceutical compositions comprising the peptides and methods of use in conditions were insufficient blood-supply occurs, or which are associated with endothelia dysfunction such as peripheral vascular diseases, coronary artery diseases, cerebrovascular diseases, diabetes and delayed wound healing, pulmonary disease, eye diseases and pathological condition related to severe infection.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com