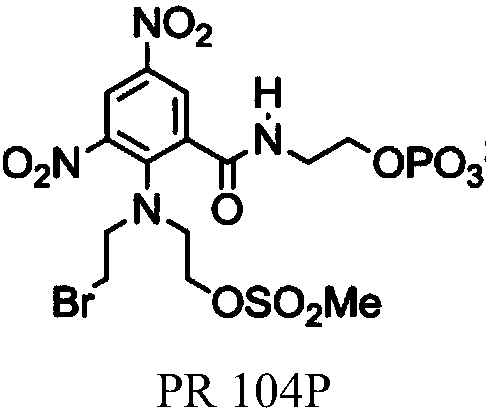
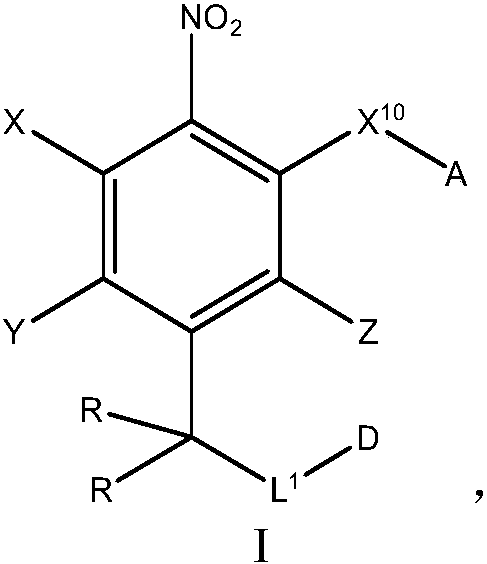
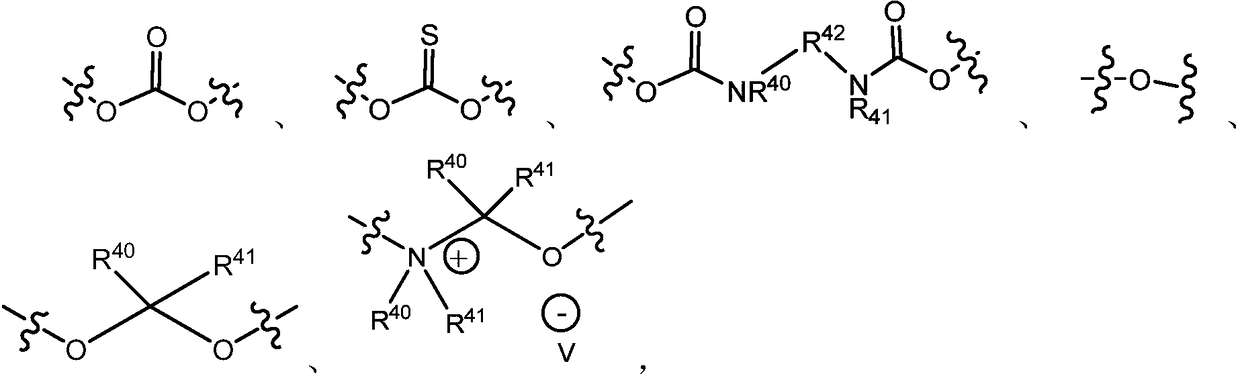
Nitrobenzyl derivatives of Anti-cancer agents
A technology of aryl and alkyl, applied in the field of cancer treatment of cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0185] Example 1: Synthesis of compounds provided herein
[0186] a.
[0187]
[0188] Compound B
[0189] Compound A (110mg) was dissolved in 5ml SOCl at 90°C 2 The solution in was heated for 3 hours. The solvent was then removed under vacuum. The residue was dissolved in toluene (5ml) and the solvent was removed again to afford compound B (112mg) without further purification.
[0190] Compound D
[0191] A solution of compound B (80 mg), compound C (2 mL) in ACN (acetonitrile, 2 ml) was heated at 80 °C overnight. Solvent was removed under vacuum. The residue was chromatographed on silica gel (ACN:H 2 O=80:20 (V / V)), to obtain 65 mg of compound D.
[0192] TH2995
[0193] Compound D (60mg) was dissolved in 5ml SOCl at 90°C 2 The solution in was heated for 4 hours. The solvent was then removed under vacuum. The residue was chromatographically separated on silica gel (AcOEt:MeOH=90:10 (V / V)) to obtain 50 mg TH2995. 1HNMR (CDCl 3 +CD 3 OD): 7.94(1H), 7.38(2H), 7...
example 2
[0213] Example 2. In Vitro Human Tumor Cell Line Cytotoxicity Analysis
[0214] In vitro proliferation data on the H460 non-cellular lung cancer human tumor cell line was obtained by exposing it to various concentrations of the compound for 2 hours, followed by a washing step and addition of fresh medium, followed by growth and cell viability staining, and compared with the measured IC of the medium-only treated control 50 Determination.
[0215] Specifically, exponentially growing cells were divided into 4 × 10 3 Cells / well were seeded in 96-well plates and incubated at 37°C in 5% CO 2 , 95% air and 100% relative humidity for 24 hours before adding the test compound. Compounds were dissolved in 100% DMSO at 200 times the desired final assay concentration. At the time of drug addition, compounds were further diluted to 4x the desired final concentration with complete medium. Aliquots of 50 μl of compounds at the indicated concentrations were added to microtiter wells alre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com