Folic acid targeting carrier loaded with mono-substituted gallocatechin gallate palmitate and its preparation method and application
A technology of gallocatechin and gallate, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of low utilization of palmitate and achieve high prolongation The time of purity, the synthesis method is simple, the effect of slowing down the oxidation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
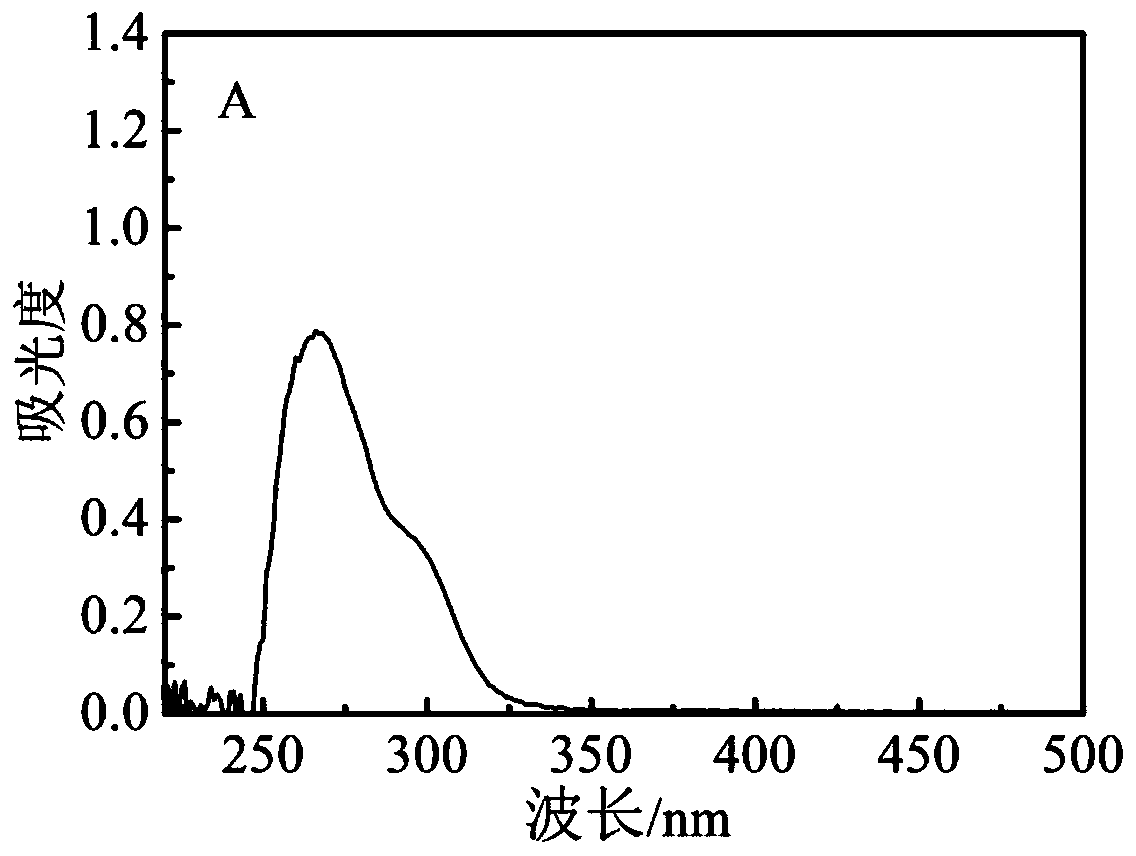
[0053] Embodiment 1: Preparation of PEGCG
[0054] (1) 10 mmol of EGCG (4.58 g) was added to 100 ml of acetone, and heated in a water bath at 40°C.
[0055] (2) After complete dissolution, 2.46 g of sodium carbonate was added to the solution.
[0056] (3) Add 20 mmol of palmitoyl chloride dropwise to the solution under stirring, and the mixture is carried out in a stoppered flask under water bath conditions.
[0057] (4) After reacting for 6 hours, the reaction mixture was filtered and washed with 100 ml of deionized water, and 100 ml of ethyl acetate was added to extract the mixture.
[0058] (5) Wash the organic phase twice with deionized water. Afterwards, the upper organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain the product PEGCG as a yellow powder.
[0059] The resulting PEGCG has the following structural formula:
[0060]
Embodiment 2
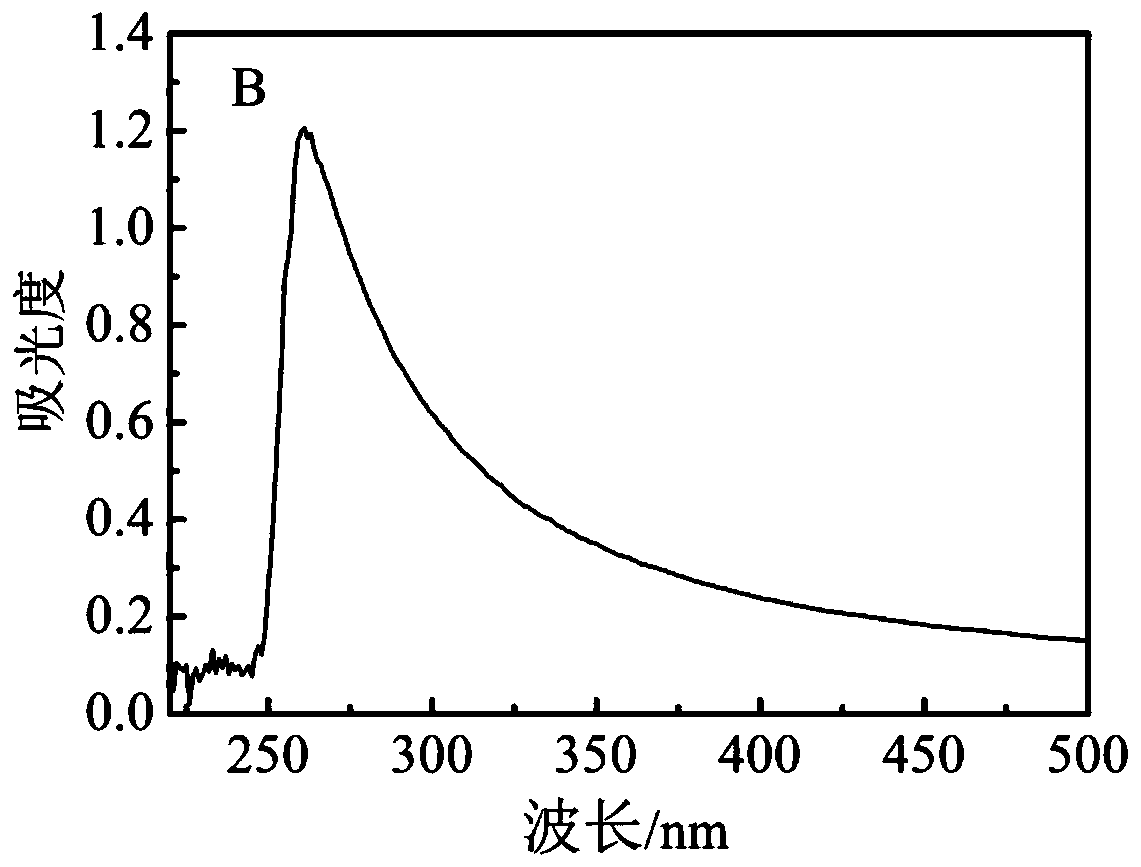
[0061] Example 2: Preparation of FA-PEG / PEGCG@ZIF-8
[0062] (1) Dissolve 40mg of monogallocatechin gallate palmitate in 4ml of methanol, mix with 0.8mL of 0.25g / mL zinc nitrate hexahydrate methanol solution, stir at room temperature for 5min, and utilize the zinc ions of zinc nitrate Form a coordination bond with monosubstituted epigallocatechin gallate palmitate;
[0063] (2) Add 10ml of 2-methylimidazole in methanol (0.2g / mL), continue stirring at room temperature for 15min, 2-methylimidazole and the zinc ion of the coordination compound form ZIF-8;
[0064] (3) Centrifuge at a rotational speed of 11000rmp for 20min, then wash three times with absolute ethanol and a deionized mixed solution to remove unreacted reagents, and dry in vacuum to obtain the PEGCG@ZIF-8 carrier;
[0065] (4) Dissolve 50mg of folic acid-polyethylene glycol into 5ml of deionized water;
[0066] (5) Add 50 mg of PEGCG@ZIF-8 carrier, ultrasonically shake evenly, and stir at room temperature for 36 h...
Embodiment 3
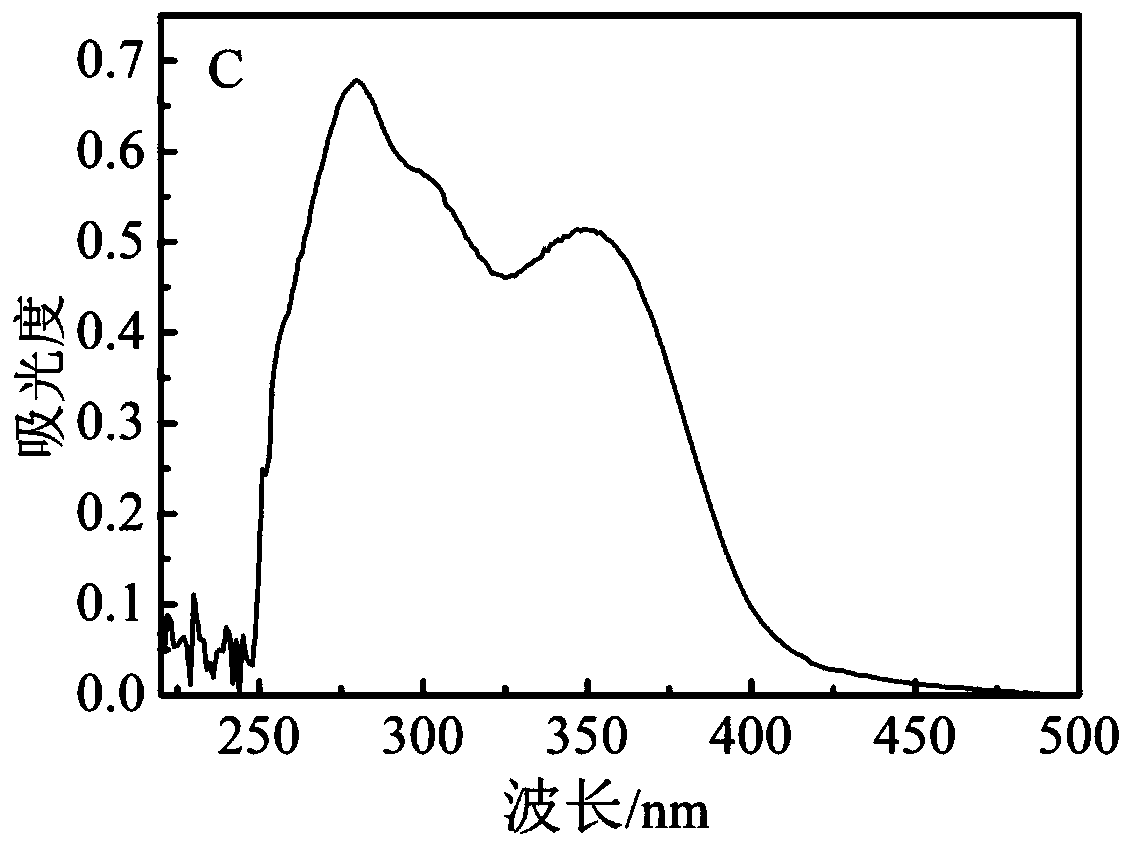
[0068] Example 3: Preparation of FA-PEG / PEGCG@ZIF-8
[0069] (1) Dissolve 10 mg of monogallocatechin gallate palmitate in 4 ml of methanol, mix with 0.8 mL of 0.25 g / mL zinc nitrate hexahydrate methanol solution, stir at room temperature for 5 min, and utilize the zinc ions of zinc nitrate Form a coordination bond with monosubstituted epigallocatechin gallate palmitate;
[0070] (2) Add 10ml of 2-methylimidazole in methanol (0.2g / mL), continue stirring at room temperature for 15min, 2-methylimidazole and the zinc ion of the coordination compound form ZIF-8;
[0071] (3) Centrifuge at a rotational speed of 11000rmp for 20min, then wash three times with absolute ethanol and a deionized mixed solution to remove unreacted reagents, and dry in vacuum to obtain the PEGCG@ZIF-8 carrier;
[0072] (4) Dissolve 50mg of folic acid-polyethylene glycol into 5ml of deionized water;
[0073] (5) Add 50 mg of PEGCG@ZIF-8 carrier, ultrasonically shake evenly, and stir at room temperature for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com