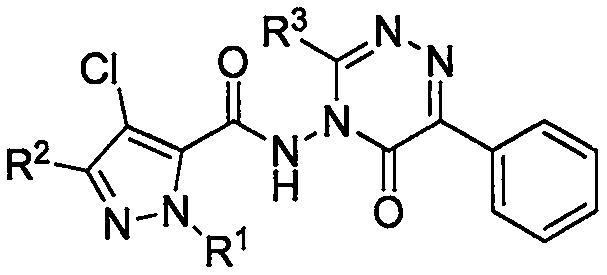
Preparation method and application of 5-pyrazol amide compound having triazinone structure
A technology of pyrazole amide and compound is applied in the field of preparation of new compounds, which can solve the problem that insecticidal activity is not reported in literature and the like, and achieve the effect of good killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 4-amino-3-alkyl-6-phenyl-1,2,4-triazin-5-(4H)-one (4.9mmol) to the reaction flask, 4-chloro-1-alkyl-3 -Alkyl-1H-pyrazole-5-carboxylic acid (4.9mmol) was dissolved in DMF, added Et 3 N (0.99g, 9.8mmol), then added EDCI (5.9mmol), HOBt (5.9mmol), reacted at 50°C for 8 hours, TLC detected that the reaction was complete. Post-treatment process: remove DMF under reduced pressure, add dichloromethane (50ml) to dilute, wash three times with water, wash once with brine, dry the dichloromethane layer with anhydrous sodium sulfate, remove dichloromethane under reduced pressure to obtain a crude product, and the crude product is chromatographed Column separation [V 石油醚 :V 乙酸乙酯 =6:1~4:1] 800mg of white crystals were obtained, m.p.92-95°C, yield 44%. 1 H NMR (400MHz, CDCl 3 )δ: 9.02(s, 1H, NH), 8.26(d, J=7.3Hz, 2H, C 6 h 5 ), 7.45-7.51 (m, 3H, C 6 h 5 ), 4.13 (s, 3H, NHCH 3 ), 2.70 (q, J=7.6Hz, 2H, CH 2 CH 3 , pyrazole), 2.64(s, 3H, CH 3 ), 1.28(t, J=7.6Hz, 3H, CH ...
Embodiment 2
[0034] Determination of insecticidal activity of 5-pyrazole amides containing triazinone structure.
[0035] Targets are armyworm, alfalfa aphid, cinnabar leafworm and rice planthopper.
[0036] Test medicament: embodiment 1 compound
[0037] Test concentration: The screening concentration is 500mg / L.
[0038] Test method: the compound of Example 1 was prepared into 5.0% EC preparation with emulsifier (Tween-80) and DMF, and diluted with distilled water to prepare a medicinal solution with the required concentration. The screening concentration is 500 mg / L, and the volume of drug treatment solution is 10 mL. With reference to the "National Southern Pesticide Creation Center Bioassay Standard Procedure", the insecticidal activity of the compound of Example 1 on armyworm (Mythima separata) was tested by the leaf dipping method.
[0039] Armyworm: soak an appropriate amount of cut corn leaf segments in the prepared medicinal solution, dry them naturally in the shade, put them ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com