Immunomodulation mixture and application
An immunomodulation and mixture technology, which can be used in drug combinations, medical preparations containing active ingredients, plant raw materials, etc., can solve problems such as unfavorable blood source and medical cost savings, large toxic and side effects of immunosuppressants, and easy relapse treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
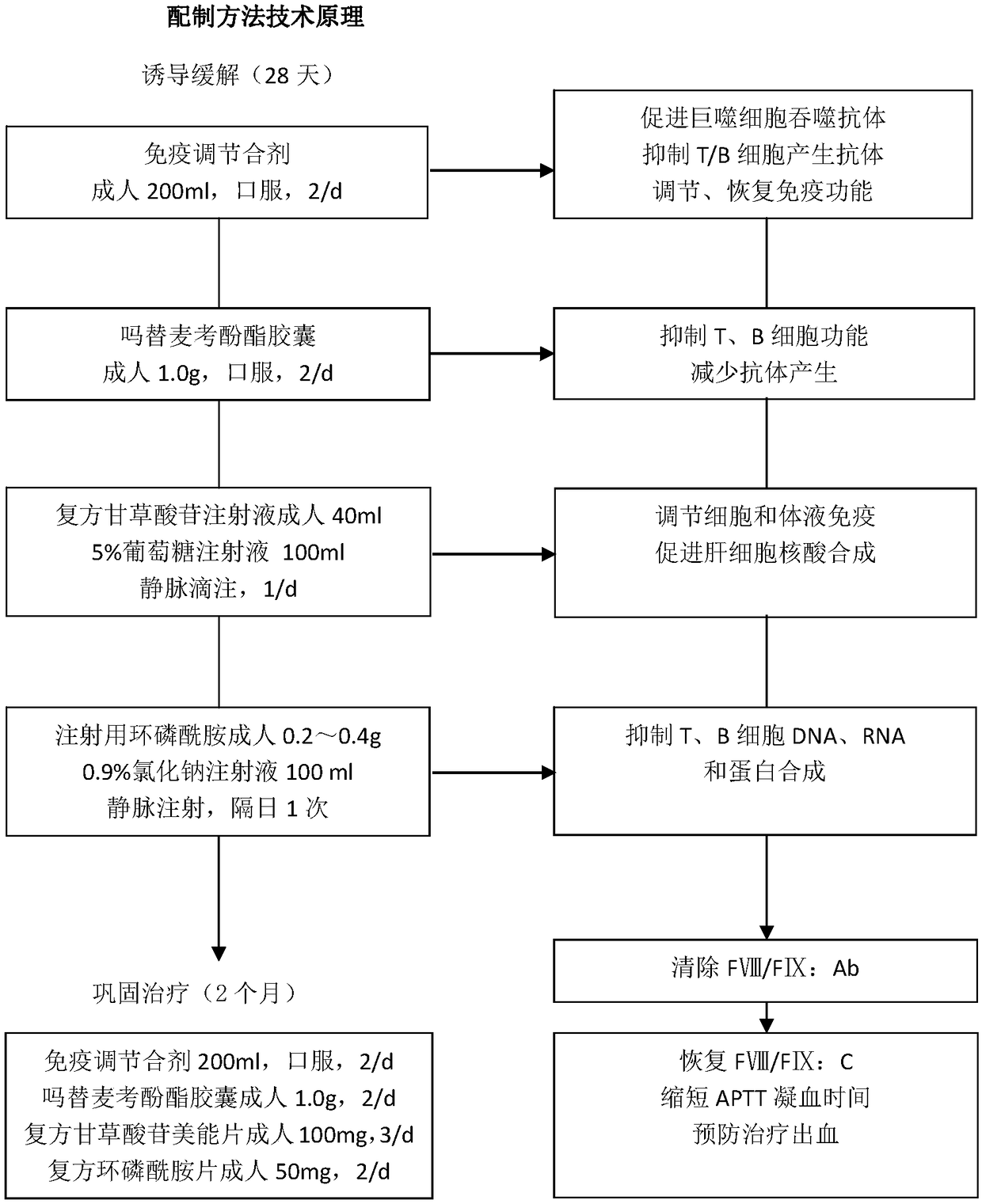
[0034] This example provides an immunomodulatory mixture, which is prepared by decocting 200ml of the following drugs: Radix Astragali: 30g, Ginseng: 10g, Atractylodes Rhizoma: 15g, Turtle Shell: 15g, Ligustrum lucidum: 30g, Angelica: 12g, Radix Paeoniae Rubra: 12g : 12g, paeonol: 12g, raw rehmannia: 15g, flavescens: 6g, rhubarb: 10g, licorice: 6g.
[0035]The preparation method of the above-mentioned immunomodulatory mixture is to soak the medicine in the formula with an appropriate amount of water for more than 2 hours, then boil it on a high fire and then change it to a low heat and boil it for 1 hour. After the medicine liquid is concentrated to 200ml, pour it out, and then add an appropriate amount of water and repeat the boil. Once, pour out the medicinal solution after it is concentrated to 200ml, discard the medicinal residues, combine and mix the medicinal solutions twice, divide into 2 bags, each bag is 200ml.
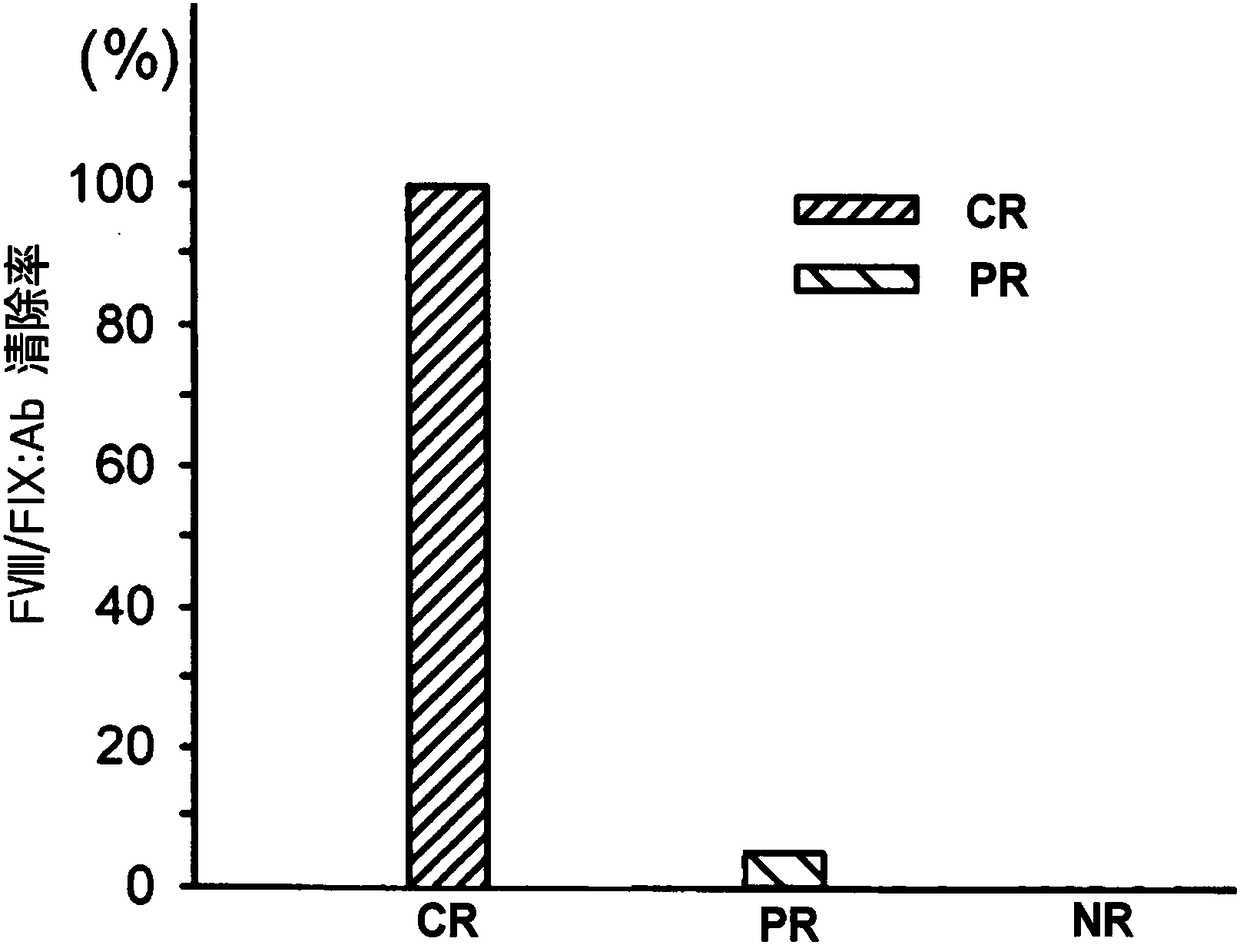
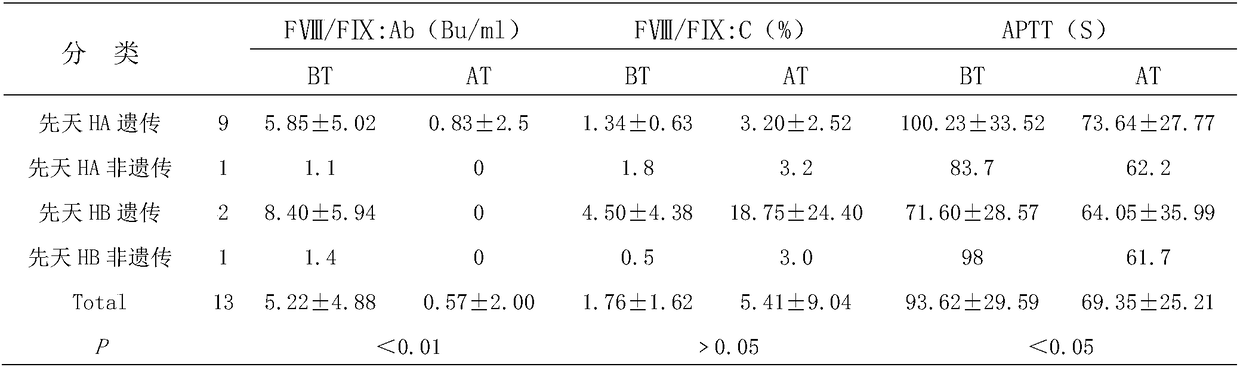
[0036] Brief Introduction of Drugs in Immunomodulatory ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com