A kind of preparation method of novel fluorocyclopentenone and its product
A technology for substituted cyclopentenone and product is applied in the field of preparation of novel fluorocyclopentenone, and achieves the effects of low energy consumption, wide range and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049] Take a 25mL round bottom flask, add 6mL methyl tert-butyl ether, enynyl ester a (64mg, 0.3mmol), (acetonitrile)[(2-biphenyl)di-tert-butylphosphine] gold (I ) (5mg, 0.006mmol), N-fluorobisbenzenesulfonamide (189mg, 0.6mmol). Stir at 500 rpm for 24 hours at normal temperature and pressure. Then use a Heidolph rotary evaporator to rotate to evaporate, the rotation speed is 200rpm, the temperature is 40°C, the vacuum degree is 0.06Mpa, and the processing time is 10min. Then through 200~300 mesh silica gel column chromatography, the eluent is ethyl acetate:petroleum ether=5:100, and the target product A (49mg, 0.26mmol, yield 86%) is separated and obtained from the appearance, signal, noise of nuclear magnetic spectrum etc. can also reflect the high purity of the product).
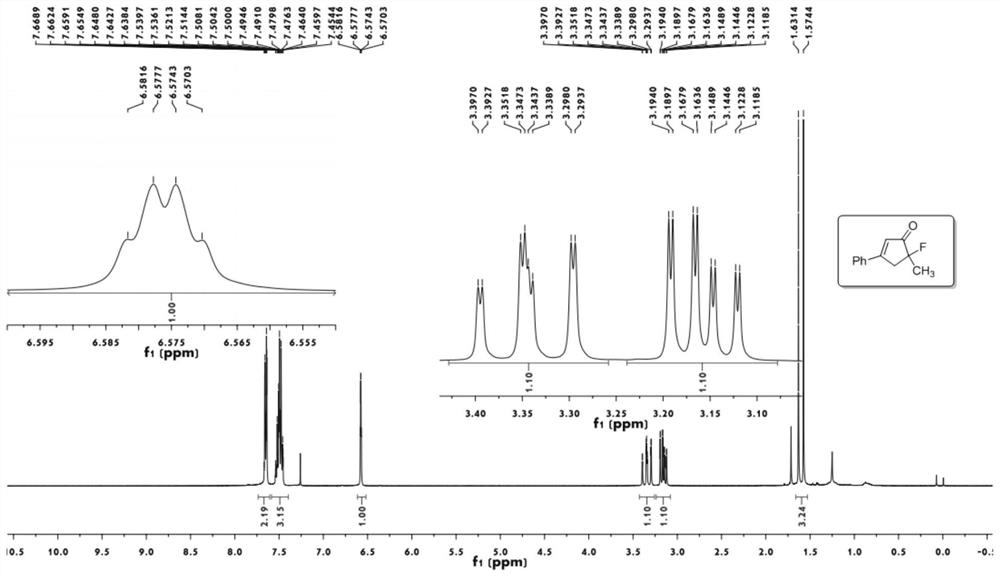
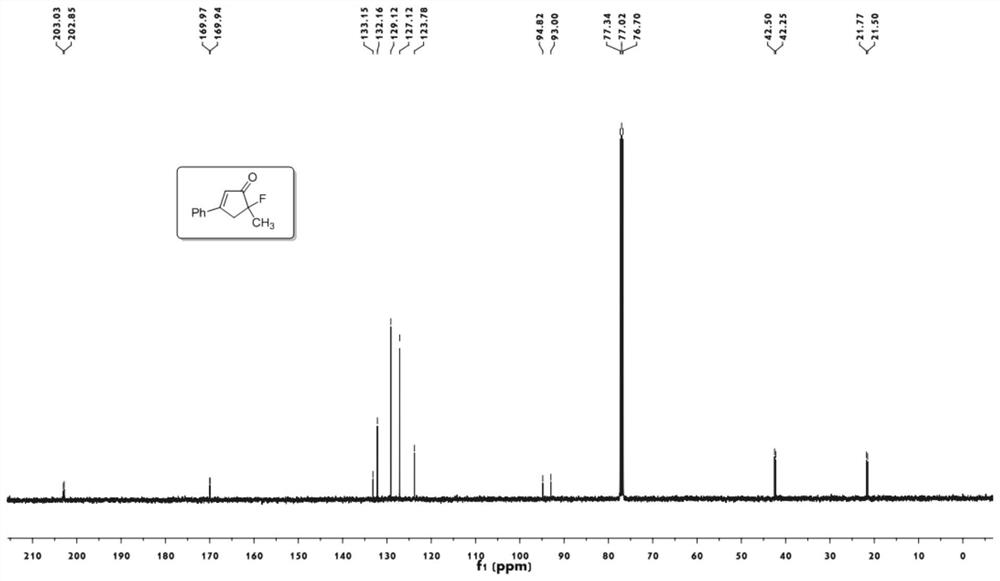
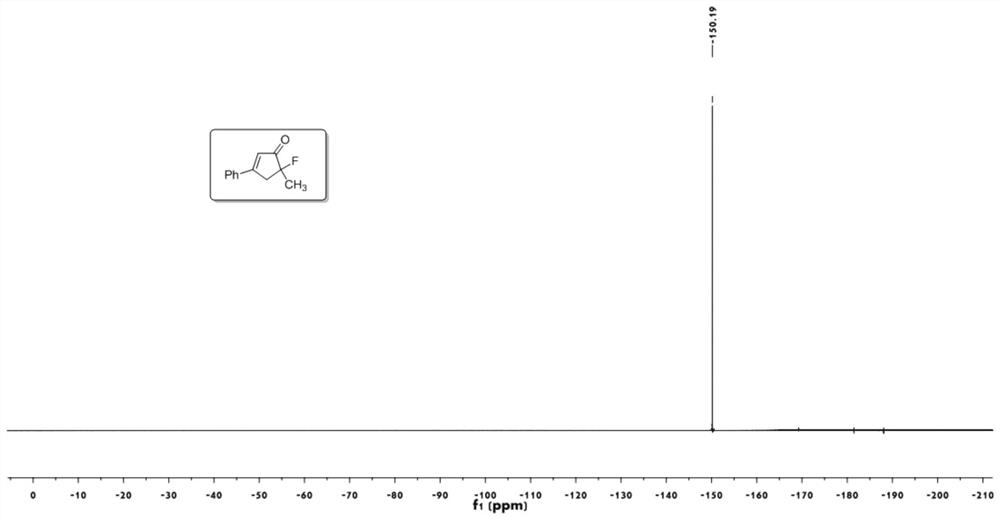
[0050] 1 H NMR (400MHz, CDCl 3 ):δ7.67-7.65(m,2H),7.55-7.46(m,3H),6.58(m,1H),
[0051] 3.41-3.30(ddd,1H,J=19.8,18.0,1.6Hz),3.20-3.12(ddd,1H,J=12.0,10.4,1.7Hz),1.61(d,3H,J=22.8Hz).
[00...
Embodiment 2
[0055]
[0056] Take a 25mL round bottom flask, add 6mL methyl tert-butyl ether, enynyl ester b (76mg, 0.3mmol), (acetonitrile)[(2-biphenyl)di-tert-butylphosphine] gold (I ) (5mg, 0.006mmol), N-fluorobisbenzenesulfonamide (189mg, 0.6mmol). Stir at 500 rpm for 24 hours at normal temperature and pressure. Then use a Heidolph rotary evaporator to rotate to evaporate, the rotation speed is 200rpm, the temperature is 40°C, the vacuum degree is 0.06Mpa, and the processing time is 10min. Then by 200~300 mesh silica gel column chromatography, the eluent is ethyl acetate:petroleum ether=5:100, and the target product B (45mg, 0.20mmol, yield 65%) is isolated and obtained from the nuclear magnetic spectrum appearance, signal, noise etc. can also reflect the high purity of the product).
[0057] 1 H NMR (400MHz, CDCl 3 ):δ7.65-7.58(m,2H),7.52-7.43(m,3H),6.47(s,1H),3.49-3.39(ddd,1H,J=21.3,8.9,6.8Hz),2.27-2.12 (m,2H),1.99-1.85(m,1H),1.85-1.73(m,1H),1.58-1.41(m,2H),1.37-1.17(m,2H). ...
Embodiment 3
[0061]
[0062] Take a 25mL round bottom flask, add 6mL methyl tert-butyl ether, enynyl ester c (146mg, 0.3mmol), (acetonitrile)[(2-biphenyl)di-tert-butylphosphine]gold(I ) (5mg, 0.006mmol), N-fluorobisbenzenesulfonamide (189mg, 0.6mmol). Stir at 500 rpm for 24 hours at normal temperature and pressure. Then use a Heidolph rotary evaporator to rotate to evaporate, the rotation speed is 200rpm, the temperature is 40°C, the vacuum degree is 0.06Mpa, and the processing time is 10min. Then through 200~300 mesh silica gel column chromatography, the eluent is ethyl acetate:petroleum ether=25:100, isolate and obtain target product C (131mg, 0.28mmol, productive rate 94%, from nuclear magnetic spectrum appearance, signal, noise etc. can also reflect the high purity of the product).
[0063] 1 H NMR (400MHz, CDCl 3 ):δ7.68-7.61(m,2H),7.59-7.43(m,5H),7.32-7.25(m,5H),7.06-7.03(m,2H),6.56(d,1H,J=1.2Hz ),3.61(t,2H,J=6.8Hz),3.26-3.06(m,2H),2.44(s,3H),2.15-2.00(m,1H),1.97-1.79(m,1H),1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com